Dichromate(VI) ions are orange in colour while chromate(VI) ions are yellow. Consider the following equilibrium.
Cr2O72−(aq) + 2OH−(aq) 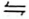 2CrO42−(aq) + H2O(l)
2CrO42−(aq) + H2O(l)
State and explain the observation that will be made if sulphuric(VI) acid is added to the mixture.