Analysis of a river water sample showed the presence of the following ions Ca2+, Na+, Cl− , NO3−.
- Name the type of water hardness present in the sample.
- Describe one precipitation method that can be used to soften the water.
- The water sample was passed through a resin as shown in the figure .
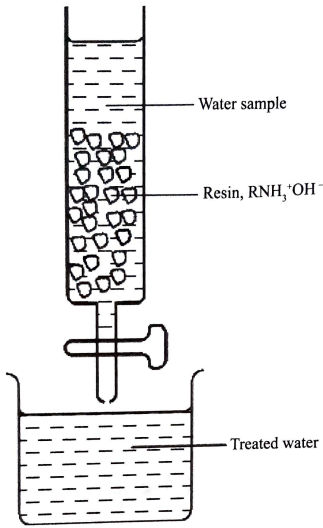
- Write an equation for a reaction that took place in the column.
- Complete treatment of the water sample required passing it through another resin. Give the formula of this resin.
- Explain why a river water sample that has been treated using resins may still require boiling to make it safe for drinking.