In the figure below, ammonia gas and an acid gas diffuse and react to form a white deposit on the walls of a long glass tube as shown.
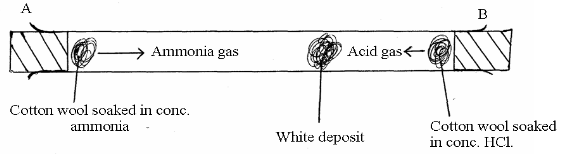
- What conclusion can be made from the result of this experiment?
- How does the size and mass of a gas affect its rate of diffusion?
- The experiment is performed at a lower temperature. Explain how the time taken to form the white deposit would be affected.