
Water
Pure water is a colourless , odorless , tasteless , neutral liquid. Pure water does not exist in nature but naturally in varying degree of purity. The main sources of water include rain, springs, borehole, lakes, seas and oceans:
Water is generally used for the following purposes:
- Drinking by animals and plants.
- Washing clothes.
- Bleaching and dyeing.
- Generating hydroelectric power.
- Cooling industrial processes.
Water dissolves many substances/solutes. It is therefore called universal solvent.
It contains about 35% dissolved Oxygen which support aquatic fauna and flora.
Water naturally exists in three phases/states solid ice, liquid water and gaseous water vapour.
The three states of water are naturally interconvertible.
The natural interconvertion of the three phases/states of water forms the water cycle.
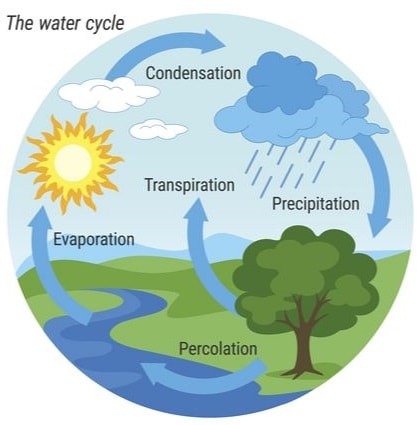
Liquid water in land, lakes, seas and oceans use the solar/sun energy to evaporate/vapourize to form water vapour/ gas. Solar/sun energy is also used during transpiration by plants and respiration by animals.
During evaporation, the water vapour rises up the earth’s surface. Temperatures decrease with height above the earth surface increase. Water vapour therefore cools as it rises up. At a height where it is cold enough to below 373Kelvin/100oC Water vapour looses enough energy to form tiny droplets of liquid.
The process by which a gas/water vapour changes to a liquid is called condensation/liquidification.
On further cooling, the liquid looses more energy to form ice/solid. The process by which a liquid/water changes to a ice/solid is called freezing/solidification. Minute/tiny ice/solid particles float in the atmosphere and coalesce/join together to form clouds. When the clouds become too heavy they fall to the earth’s surface as rain/snow as the temperature increase with the fall.
Pure water has:
- fixed/constant/sharp freezing point/melting point of 273K /0oC
- fixed/constant/sharp boiling point of 373K /100oC at sea level /1 atmosphere pressure
- fixed density of 1gcm-3
This is the criteria of identifying pure/purity of water.
Whether a substance is water can be determined by using the following methods:
Tests for Presence of Water
To test for presence of water using anhydrous copper (II) suphate (VI)
Procedure
- Put about 2g of anhydrous copper (II) sulphate crystals into a clean test tube. Add three drops of tap water.
- Repeat the procedure using distilled water.
Observation
Colour changes from white to blue
Explanation
Anhydrous copper (II) sulphate is white. On adding water, anhydrous copper (II) sulphate (VI) gains/reacts with water to form hydrated copper (II) sulphate.
Hydrated copper (II) sulphate is blue . Hydrated copper (II) sulphate contains water of crystallization.
The change of white anhydrous copper (II) sulphate (VI) to blue hydrated copper (II) sulphate (VI) is a confirmatory test for the presence of water.
Chemical equation
Anhydrous copper (II) sulphate + Water → hydrated copper (II) sulphate
CuSO4(s) + 5H2O(l) → CuSO4.5H2O(s)
To test for presence of water using anhydrous cobalt (II) chloride
Procedure
- Put about 5cm3 of water into a clean test tube.
- Dip a dry anhydrous cobalt (II) chloride paper into the test tube.
- Repeat the procedure using distilled water.
Observation
Colour changes from blue to pink
Explanation
Anhydrous cobalt (II) chloride is blue. On adding water, anhydrous cobalt (II) chloride gains/reacts with water to form hydrated cobalt (II) chloride.
Hydrated cobalt (II) chloride is pink.
Hydrated cobalt (II) chloride contains water of crystallization.
The change of blue anhydrous cobalt (II) chloride to pink hydrated cobalt (II) chloride is a confirmatory test for the presence of water Chemical equation.
Anhydrous cobalt (II) chloride + Water -> Hydrated cobalt (II) chloride
CoCl2 (s) + 5H2O (l) → CoCl2.5H2O(s)
Burning a candle in air
Most organic substances/fuels burn in air to produce water. Carbon (IV) oxide gas is also produced if the air is sufficient/excess.
Procedure
Put about 2g of anhydrous copper (II) sulphate crystals in a boiling tube.
Put about 5cm3 of lime water in a boiling tube.
Light a small candle stick. Place it below an inverted thistle/filter funnel.
Collect the products of the burning candle by setting the apparatus as below.
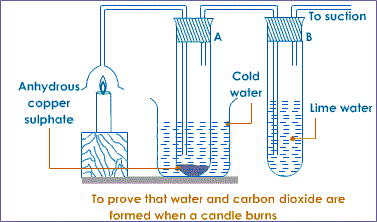
Observation
The sunction pump pulls the products of burning into the inverted funnel. Colour of anhydrous copper (II) sulphate (VI) changes from white to blue. A white precipitate is formed in the lime water/calcium hydroxide.
Explanation
When a candle burn it forms a water and carbon (IV) oxide.
Water turns anhydrous copper (II) sulphate (VI) changes from white to blue.
Carbon (IV) oxide gas forms white precipitate when bubbled in lime water/calcium hydroxide.
Since:
- hydrogen in the wax burn to form water
Hydrogen + Oxygen → Water
2H2(g) + O2(g) → 2H2O(g/l) - carbon in the wax burn to form carbon (IV) oxide
Hydrogen + Oxygen → Water
C(s) + O2 (g) → CO2 (g)
The candle before burning therefore contained Carbon and Hydrogen only. A compound made up of hydrogen and carbon is called Hydro carbon.
A candle is a hydrocarbon.
Other hydrocarbons include: Petrol, diesel, Kerosene, and Laboratory gas. Hydrocarbons burn in air to form water and carbon (IV) oxide gas.
Hydrocarbons + Oxygen → Water + Oxygen
Water pollution
Water pollution takes place when undesirable substances are added into the water. Sources of water pollution include:
- Industrial chemicals being disposed into water bodies like rivers, lakes and oceans.
- Discharging untreated /raw sewage into water bodies.
- Leaching of insecticides/herbicides form agricultural activities into water bodies.
- Discharging non-biodegradable detergents after domestic and industrial use into water bodies.
- Petroleum oil spilling by ships and oil refineries
- Toxic/poisonous gases from industries dissolving in rain.
- Acidic gases from industries dissolving in rain to form “acid rain”
- Discharging hot water into water bodies. This reduces the quantity of dissolved Oxygen in the water killing the aquatic fauna and flora.
Water pollution can be reduced by:
- Reducing the use of agricultural fertilizers and chemicals in agricultural activities.
- Use of biological control method instead of insecticides and herbicides
- Using biodegradable detergents
Reaction of water with metals
Some metals react with water while others do not. The reaction of metals with water depends on the reactivity series. The higher the metal in the reactivity series the more reactive the metal with water.
The following experiments shows the reaction of metals with cold water and water vapour/steam.
Reaction of sodium/ potassium with cold water:
Procedure
- Put about 500cm3 of water in a beaker.
- Add three drops of phenolphthalein indicator/litmus solution/universal indicator solution/methyl orange indicator into the water.
- Cut a very small piece of sodium.
- Using a pair of forceps put the metal into the water.
Observation
- Sodium melts to a silvery ball that floats and darts on the surface decreasing in size.
- Effervescence/fizzing/ bubbles of colourless gas produced.
- Colour of phenolphthalein turns pink
- Colour of litmus solution turns blue
- Colour of methyl orange solution turns Orange
- Colour of universal indicator solution turns blue
Explanation
Sodium is less dense than water. Sodium floats on water and vigorously reacts to form an alkaline solution of sodium hydroxide and producing hydrogen gas. Sodium is thus stored in paraffin to prevent contact with water.
Chemical equation
Sodium + Water → Sodium hydroxide + Hydrogen gas
2Na(s) + 2H2O (l) → 2NaOH (aq) + H2 (g)
To collect hydrogen gas, Sodium metal is forced to sink to the bottom of the trough/beaker by wrapping it in wire gauze/mesh.
Potassium is more reactive than Sodium. On contact with water it explodes /burst into flames. An alkaline solution of potassium hydroxide is formed and hydrogen gas.
Chemical equation
Potassium + Water → Potassium hydroxide + Hydrogen gas
2K(s) + 2H2O (l) → 2KOH (aq) + H2 (g)
Caution: Reaction of Potassium with water is very risky to try in a school laboratory.
Reaction of Lithium/ Calcium with cold water:
Procedure
- Put about 200cm3 of water in a beaker.
- Add three drops of phenolphthalein indicator/litmus solution/universal indicator solution/methyl orange indicator into the water.
- Cut a small piece of Lithium.
- Using a pair of forceps put the metal into the water.
- Repeat with a piece Calcium metal
Observation
- Lithium sinks to the bottom of the water.
- Rapid effervescence/fizzing/ bubbles of colourless gas produced.
- Colour of phenolphthalein turns pink
- Colour of litmus solution turns blue
- Colour of methyl orange solution turns Orange
- Colour of universal indicator solution turns blue
Explanation
Lithium and calcium are denser than water. Both sink in water and vigorously react to form an alkaline solution of Lithium hydroxide / calcium hydroxide and producing hydrogen gas.
Lithium is more reactive than calcium. It is also stored in paraffin like Sodium to prevent contact with water.
Chemical equation
Lithium + Water → Lithium hydroxide + Hydrogen gas
2Li(s) + 2H2O (l) → 2LiOH (aq) + H2 (g)
Calcium + Water → Calcium hydroxide + Hydrogen gas
Ca(s) + 2H2O (l) → Ca(OH)2 (aq) + H2 (g)
Reaction of Magnesium/Zinc/ Iron with Steam/water vapour:
Procedure method1
- Place some wet sand or cotton/glass wool soaked in water at the bottom of an ignition/hard glass boiling tube.
- Polish magnesium ribbon using sand paper.
- Coil it at the centre of the ignition/hard glass boiling tube.
- Set up the apparatus as below.
- Heat the wet sand or cotton/glass wool soaked in water gently to:
- Drive away air in the ignition/hard glass boiling tube.
- Generate steam
- Heat the coiled ribbon strongly using another burner. Repeat the experiment using Zinc powder and fresh Iron
filings.
Set up of apparatus
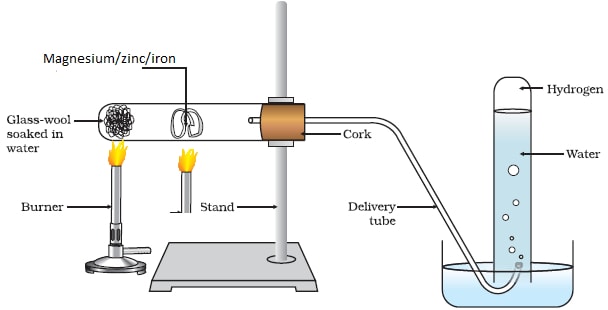
Observations
With Magnesium ribbon:
- The Magnesium glows with a bright flame (and continues to burn even if heating is stopped)
- White solid /ash formed
- White solid /ash formed dissolve in water to form a colourless solution
- Colourless gas produced/collected that extinguish burning splint with “pop sound”
With Zinc powder:
- The Zinc powder turns red hot on strong heating.
- Yellow solid formed that turn white on cooling.
- White solid formed on cooling does not dissolve in water.
With Iron fillings:
- The Iron fillings turn red hot on strong heating
- Dark blue solid formed
- Dark blue solid formed does not dissolve in water.
Procedure method 2
- Put some water in a round bottomed flask
- Polish magnesium ribbon using sand paper.
- Coil it at the centre of a hard glass tube
- Set up the apparatus as below.
- Heat water strongly to boil so as to:
- drive away air in the glass tube.
- generate steam
- Heat the coiled ribbon strongly using another burner. Repeat the experiment using Zinc powder and fresh Iron filings.
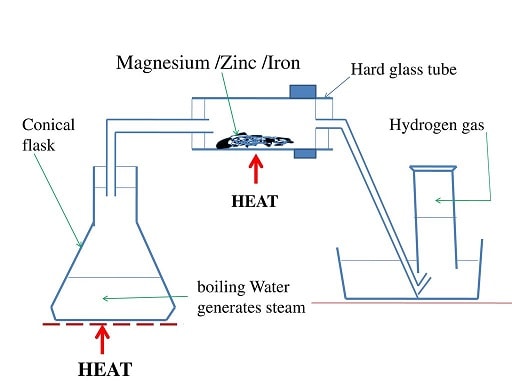
Observations
With Magnesium ribbon:
- The Magnesium glows with a bright flame (and continues to burn even if heating is stopped)
- White solid/ash formed
- White solid /ash formed dissolve in water to form a colourless solution Colourless gas produced/collected that extinguish burning splint with “pop sound”.
With Zinc powder:
- The Zinc powder turns red hot on strong heating
- Yellow solid formed that turn white on cooling
- White solid formed on cooling does not dissolve in water.
With Iron fillings:
- The Iron fillings turn red hot on strong heating
- Dark blue solid formed
- Dark blue solid formed does not dissolve in water.
Explanations
Hot magnesium burn vigorously in steam. The reaction is highly exothermic generating enough heat/energy to proceed without further heating.
White Magnesium oxide solid/ash is left as residue.
Hydrogen gas is produced. It extinguishes a burning splint with a “pop sound”.
Chemical Equation
Magnesium + Steam → Magnesium oxide + Hydrogen
Mg(s) + H2O( g ) → MgO(s) + H2(g)
Magnesium oxide reacts /dissolves in water to form an alkaline solution of Magnesium oxide
Chemical Equation
Magnesium oxide + Water → Magnesium hydroxide
MgO(s) + H2O(l) → Mg(OH)2 (aq)
Hot Zinc react vigorously in steam forming yellow Zinc oxide solid/ash as residue which cools to white.
Hydrogen gas is produced. It extinguishes a burning splint with a “pop sound”.
Chemical Equation
Zinc + Steam → Zinc oxide + Hydrogen
Zn(s) + H2O( g ) → ZnO(s) + H2 (g)
Zinc oxide does not dissolve in water.
Hot Iron reacts with steam forming dark blue tri iron tetra oxide solid/ash as residue.
Hydrogen gas is produced.
It extinguishes a burning splint with a “pop sound”.
Chemical Equation
Iron + Steam → Tri iron tetra oxide + Hydrogen
2Fe(s) + 4H2O( g → Fe2O4 (s) + 4H2 (g)
Tri iron tetra oxide does not dissolve in water.
Aluminum reacts with steam forming an insoluble coat /cover of impervious layer of aluminum oxide on the surface preventing further reaction.
Lead, Copper, Mercury, Silver, Gold and Platinum do not react with either water or steam.

Hydrogen
Occurrence
Hydrogen does not occur free in nature. It occurs as Water and in Petroleum.
School laboratory Preparation
Procedure
- Put Zinc granules in a round/flat/conical flask.
- Add dilute sulphuric (VI) /Hydrochloric acid.
- Add about 3cm3 of copper (II) sulphate (VI) solution.
- Collect the gas produced over water as in the set up below.
- Discard the first gas jar. Collect several gas jars.
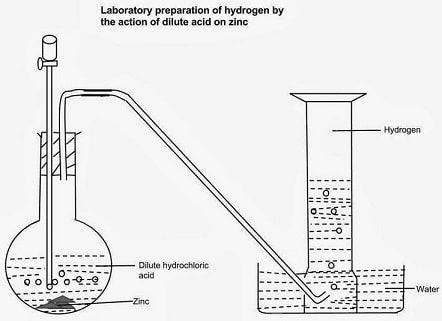
Observation/Explanation
Zinc reacts with dilute sulphuric (VI)/hydrochloric acid to form a salt and produce hydrogen gas.
When the acid comes into contact with the metal, there is rapid effervescence/ bubbles /fizzing are produced and a colourless gas is produced that is collected:
- Over water because it is insoluble in water
- Through downward displacement of air/upward delivery because it is less dense than air.
The first gas jar is impure. It contains air that was present in the apparatus.
Copper (II) sulphate (VI) solution act as catalyst.
Chemical equation
Zinc + Hydrochloric acid → Zinc chloride + Hydrogen
Zn(s) + 2HCl (aq) → ZnCl2 (aq) + H2 (g)
Ionic equation
Zn (s) + 2H+ (aq) → Zn2+ (aq) + H2 (g)
Chemical equation
Zinc + Sulphuric (VI) acid → Zinc Sulphate (VI) + Hydrogen
Zn(s) + H2SO4 (aq) → ZnSO4 (aq) + H2 (g)
Ionic equation
Zn(s) + 2H+ (aq) → Zn2+ (aq) + H2 (g)
Chemical equation
Magnesium + Hydrochloric acid -> Magnesium chloride + Hydrogen
Mg(s) + 2HCl (aq) → MgCl2 (aq) + H2 (g)
Ionic equation
Mg(s) + 2H+ (aq) → Mg2+ (aq) + H2 (g)
Chemical equation
Magnesium + Sulphuric (VI) acid -> Magnesium Sulphate(VI) + Hydrogen
Mg(s) + H2SO4 (aq) → MgSO4 (aq) + H2 (g)
Ionic equation
Mg (s) + 2H+ (aq) → Mg2+ (aq) + H2 (g)
Chemical equation
Iron + Hydrochloric acid → Iron (II) chloride + Hydrogen
Fe(s) + 2HCl (aq) → FeCl2 (aq) + H2 (g)
Ionic equation
Fe(s) + 2H+ (aq) → Fe2+ (aq) + H2 (g)
Chemical equation
Iron + Sulphuric (VI) acid → Iron (II) Sulphate (VI) + Hydrogen
Fe(s) + H2SO4 (aq) → FeSO4 (aq) + H2 (g)
Ionic equation
Fe(s) + 2H+ (aq) → Fe2+ (aq) + H2 (g)
Note
Hydrogen cannot be prepared from reaction of:
- Nitric (V) acid and a metal. Nitric (V) acid is a strong oxidizing agent. It oxidizes hydrogen gas to water.
- Dilute sulphuric (VI) acid with calcium/Barium/Lead because Calcium sulphate (VI), Barium sulphate (VI)
- and Lead (II) sulphate (VI) salts formed are insoluble. Once formed, they cover/coat the unreacted calcium/Barium/Lead stopping further reaction and producing very small amount/volume of hydrogen gas.
- Dilute acid with sodium/potassium. The reaction is explosive.
Properties of Hydrogen gas
Physical properties
- Hydrogen is a neutral, colourless and odorless gas. When mixed with air it has a characteristic pungent choking smell
- It is insoluble in water thus can be collected over water.
- It is the lightest known gas. It can be transferred by inverting one gas jar over another.
Chemical properties
Burning
- Hydrogen does not support burning/combustion. When a burning splint is inserted into a gas jar containing Hydrogen, the flame is extinguished /put off.
- Pure dry hydrogen burn with a blue quiet flame to form water. When a stream of pure dry hydrogen is ignited, it catches fire and continues to burn with a blue flame.
- Impure (air mixed with) hydrogen burns with an explosion. Small amount/ volume of air mixed with hydrogen in a test tube produce a small explosion as a “pop” sound. This is the confirmatory test for the presence of Hydrogen gas. A gas that burns with a “pop” sound is confirmed to be Hydrogen.
Redox
When a stream of dry hydrogen gas is passed through black copper (II) oxide, hydrogen gas gains the oxygen from copper (II) oxide.
Black copper (II) oxide is reduced to brown copper metal.
Black copper (II) oxide thus the Oxidizing agent.
Hydrogen gas is oxidized to Water. Hydrogen is the Reducing agent.
Set up of apparatus.
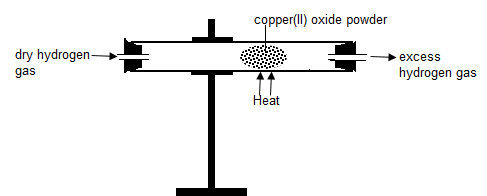
Chemical equation
- In glass tube
Copper (II) Oxide + Hydrogen → Copper + Hydrogen gas
CuO (s) + H2 (g) → Cu(s) + H2O(l) - when excess Hydrogen is burning.
Oxygen + Hydrogen → Water
O2 (g) + 2H2 (g) → 2H2O (l)
Chemical equation
- In glass tube
Lead (II) Oxide + Hydrogen → Lead + Hydrogen gas
PbO (s) + H2 (g) → Pb(s) + H2O (l) - when excess Hydrogen is burning.
Oxygen + Hydrogen → Water
O2 (g) + 2H2 (g) → 2H2O (l)
Chemical equation
- In glass tube
Iron (III) Oxide + Hydrogen → Iron + Hydrogen gas
Fe2O3 (s) + 3H2 (g) → Fe(s) + 3H2O (l) - when excess Hydrogen is burning.
Oxygen + Hydrogen → Water
O2 (g) + 2H2 (g) → 2H2O (l)
Water as an Oxide of Hydrogen
Burning is a reaction of an element with Oxygen. The substance formed when an element burn in air is the oxide of the element. When hydrogen burns, it reacts/ combines with Oxygen to form the oxide of Hydrogen.
The oxide of Hydrogen is called water. Hydrogen is first dried because a mixture of Hydrogen and air explodes. The gas is then ignited. The products condense on a cold surface/flask containing a freezing mixture.
A freezing mixture is a mixture of water and ice.
The condensed products are collected in a receiver as a colourless liquid.
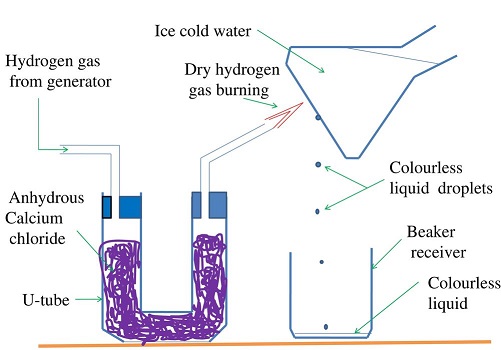
Tests
- When about 1g of white anhydrous copper (II) sulphate (VI) is added to a sample of the liquid, it turns to
blue. This confirms the liquid formed is water. - When blue anhydrous cobalt (II) chloride paper is dipped in a sample of the liquid, it turns to pink . This confirms the liquid formed is water.
- When the liquid is heated to boil, its boiling point is 100oC at sea level/one atmosphere pressure. This confirms the liquid is pure water.
Uses of Hydrogen gas
- Hydrogenation/Hardening of unsaturated vegetable oils to saturated fats/margarine.
When Hydrogen is passed through unsaturated compounds in presence of Nickel catalyst and about 150oC , they become saturated. Most vegetable oil is unsaturated liquids at room temperature. They become saturated and hard through hydrogenation. - In weather forecast balloons.
Hydrogen is the lightest known gas. Meteorological data is collected for analysis by sending hydrogen filled weather balloons to the atmosphere. The data collected is then used to forecast weather conditions. - In the Haber process for the manufacture of Ammonia
Hydrogen is mixed with Nitrogen in presence of Iron catalyst to form Ammonia gas. Ammonia gas is a very important raw material for manufacture of agricultural fertilizers. - In the manufacture of Hydrochloric acid.
Limited volume/amount of Hydrogen is burnt in excess chlorine gas to form Hydrogen chloride gas. Hydrogen chloride gas is dissolved in water to form Hydrochloric acid. Hydrochloric acid is used in pickling/washing metal surfaces. - As rocket fuel.
Fixed proportions of Hydrogen and Oxygen when ignited explode violently producing a lot of energy/heat. This energy is used to power/propel a rocket to space. - In oxy-hydrogen flame for welding.
A cylinder containing Hydrogen when ignited in pure Oxygen from a second cylinder produces a flame that is very hot. It is used to cut metals and welding.
Download WATER AND HYDROGEN - Chemistry Notes Form 1.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

