Questions
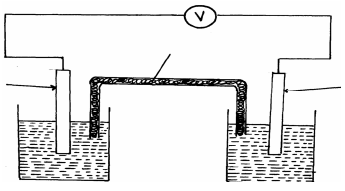
- The setup below was used to carry out the electrolysis of Magnesium sulphate solution using inert electrodes.
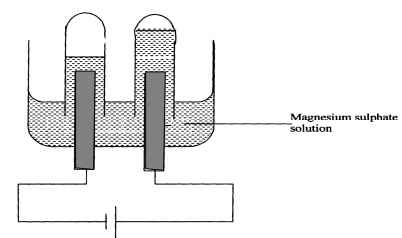
- Name a suitable pair of electrode that can be used in the above process.
- State and explain the changes on the concentration of magnesium sulphate solution as the process proceeds.
- During purification of copper by electrolysis, 1.48g of copper were deposited when a current was passed through aqueous copper (II) sulphate for 2½ hours. Calculate the amount of current passed. (Cu = 63.5 1Faraday = 96500C)
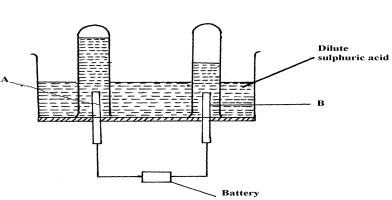
- The diagram below represents a set-up that can be used for the electrolysis of dilute sulphuric acid
- Name the electrodes A and B
- Write an equation for the reaction taking place at electrode B
- What happens to the concentration dilute sulphuric acid as the reaction continues?
- In an electrolysis, a current of 200A was passed through molten oxide of metal Q for 58 minutes and 64.8g of the metal deposited. Determine;
- Charge on metal Q
- The volume of oxygen gas produced at standard temperature and pressure Q = 27 IF = 96500C, molar gas volume stp =22.4dm3
- Consider the reduction potentials below.
Pb2+(aq) + 2e- → Pb(s) = -0.13V
Mg2+(aq) + 2e- → Mg(s) = -0.76V- Write the overall Redox reaction that takes place when the above half cells are connected.
- Determine the Eθ value of the above cell.
- Calculate which group of the periodic table is element F?
- An oxide of element F has the following formula:- F2O5
Determine the oxidation state of F
Element Sodium Magnesium Aluminium Atomic number 11 12 13 - The table below gives elements and their atomic numbers. Answer the questions that follow:
Compare the electrical conductivity of sodium and aluminium. Explain - What mass of Zinc will be deposited from a solution of Zinc (II) Chloride when a current of 3A is passed through the Zinc (II) Chloride solution during electrolysis for 50minutes? (Zn= 65, 1 Faraday = 96500C)
- Study the flow chart below and answer the questions that follow:
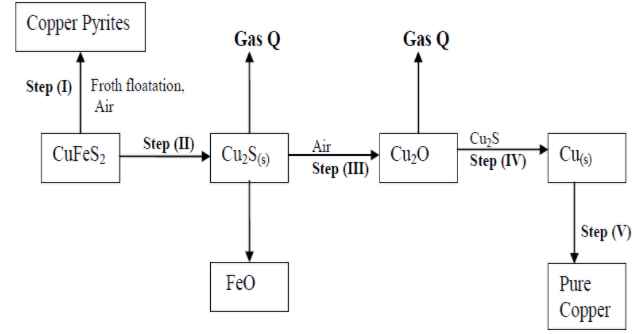
- Name gas Q …………………………………………………………… .
- With the help of diagram, describe how step (V) is carried out
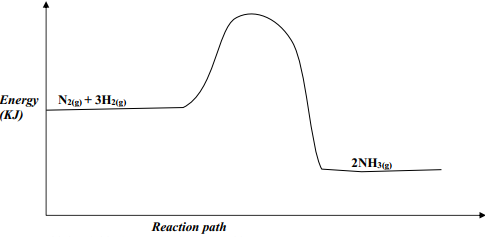
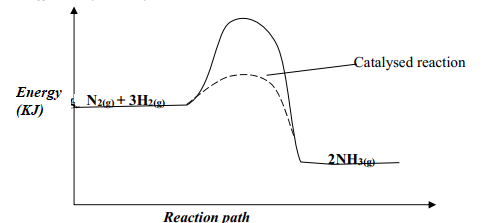
- Nitrogen and hydrogen react reversibly according to the equation:-
N2(g) + 3H2(g) ⇌ 2NH3(g); ΔH = -92 KJmol-1
The energy level diagram for the above reaction is shown below:-- How would the yield of ammonia be affected by:
- A decrease in temperature
- An increase in pressure
- How does a catalyst affect reversible reaction already in equilibrium?
- On the above diagram, sketch the energy level diagram that would be obtained when iron catalyst is added to the reaction
- How would the yield of ammonia be affected by:
- Study the electrode potentials in the table below and answer the question that follow:
(Letters are not the actual symbols of elements)
(Eθ/Volts)
H2+(aq) + 2 e- → H(s) +0.34
Z2+(aq) + 2e- → Z(s) -2.38
G+(aq) + e- → G (s) +0.80
T2+(aq) + 2e- → T(s) - 2.87- Which one is the strongest reducing agent?
- Write the ionic equation for the reaction that takes place when Z is dipped in a solution of G+ ions
- Calculate the Eθ cell value of the reaction in 11(b) above
- When a hydrocarbon was completely burnt in oxygen, 4.2g of Carbon (IV) oxide and 1.71g of water were formed. Determine the empirical of the hydrocarbon. (H=10 C=12.0 O=16.0)
- During electrolysis of aqueous copper (II) sulphate 144,750 coulombs of electricity were used.
Calculate the mass of copper metal that was obtained (Cu =64 1Faraday = 96,5000 coulombs) - Sodium metal reacts with oxygen according to the following equation:-
heat
6Na(s) + 2O2(g) → Na2O2(s) + 2Na2O(s)
State one physical and one chemical difference between Na2O2 and Na2O
Physical difference ……………………………………………
Chemical difference…………………………………… - The diagram below shows an electrochemical cell:
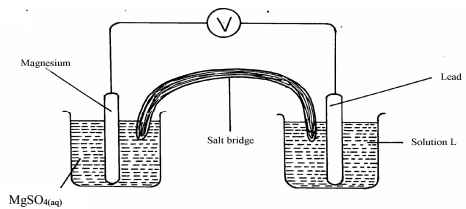
- Give the formula of the possible salt L
- On the diagram show the direction of movement of electrons
- Write the cell representation
- The reaction below is a redox reaction
MnO4-(aq) + 8H+(aq) + 5Fe2+(aq) →- Identify the species reduced. Explain
- Write the equation for the oxidation reaction
- Consider the cell diagram below
Cr(s)/Cr3+(aq) // Fe2+(aq)/Fe(s) Eθ = + 0.30V- Write the overall cell reaction for the above electrochemical cell
- Given that Eθ value for Fe2+(aq)/Fe(s) is -0.40V,calculate the Eθ value for Cr3+(aq)/Cr(s)
-
- Describe the process by which Trichloro fluoromethane Nitrogen is obtained from air on a large scale
- Study the flow chart below and answer the questions that follow
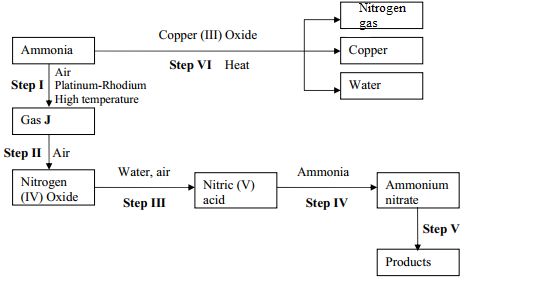
- Identify gas J
- Using oxidation numbers, show that ammonia is the reducing agent in step VI
- Write the equation that occurs in step V
- Give one use of ammonium nitrate
- The table below shows the observations made when aqueous ammonia was added to cations of elements E, F and G until in excess
Cation of Addition of a few drops of
aqueous ammoniaAddition of excess
aqueous ammoniaE White precipitate Insoluble F No precipitate No precipitate G White precipitate Dissolves - Select the cation that is likely to be Zn2+ ………………………………
- Given that the formula of the cation of element E is E2+, write the ionic equation for the reaction between E2+ and aqueous ammonia
- Study the standard electrode potential for the half-cells given below and answer the questions that follow.(The letter do not represent the actual symbols of the elements)
Eθ Volts
N+(aq) + e- → N(s) ; -2.92
J+(aq) + e- → J(s) ; +0.52
K+(aq) + e- → ½ K2(g); 0.00
½G2(g) + e- → G-(aq); +1.36
M2+(aq) + 2e- → M(s); -0.44- Identify the strongest oxidizing agents. Give a reason for your answer
- Which two half-cells would produce the highest potential difference when combined?
- In the space below draw a complete electro chemical cell of the two-half cells mentioned in (ii) above
-
- Below is a simplified diagram of the Down’s cell for the extraction of sodium. Study it and answer the question that follow:-
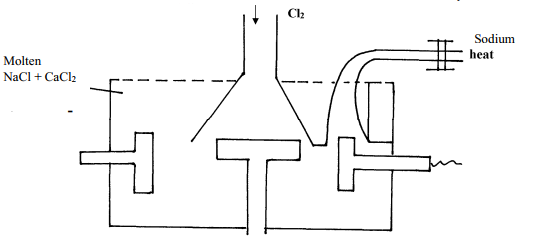
- From which substances are the electrodes made?
Cathode…………………………………………………………….
Anode…………………………………………………………………… - State and explain why sodium chloride is mixed with calcium chloride
- What is the role of the iron gauze
- Write equations for the reaction at :-
cathode…………………………………………………………….
anode……………………………………………………………. - Which property of sodium makes it possible to collect it as shown?
- From which substances are the electrodes made?
- When a current of 6.42 A was passed through an electrolyte Y2+ ions for 10minutes, 2.74 of Y were deposited
- Calculate the quantity of electricity passed in the experiment
- Determine the relative atomic mass of Y (1 Faraday = 96000 coulombs)
- Below is a simplified diagram of the Down’s cell for the extraction of sodium. Study it and answer the question that follow:-
-
- The table gives the standard redox potentials for a number of half reactions. Use it to answer the questions that follow:-
(Eθ/Volts)
Zn2+(aq) + 2e- ⇌ Zn(s) -0.76
Fe2+(aq) + 2e- ⇌ Fe(s) -0.44
I2+(l) + 2e- ⇌ 2I- (aq) +0.54
Fe3+(aq) + e- ⇌ Fe2+(aq) +0.77
Ag+(aq) + e- ⇌ Ag(s) +0.88- Relative to which half-cell reaction are the above electrode potentials expressed?
- Calculate the e.m.f of the cell made up by combining the I2(l)/2I-(aq) electrode and Zn2+(aq)/Zn(s) electrode
- Which of the substances listed in the above table is :-
- The strongest oxidising agent
- The strongest reducing agent
- Which substances could be used to convert iodide ions to iodine? Write balanced equations for any possible conversions
- The table gives the standard redox potentials for a number of half reactions. Use it to answer the questions that follow:-
-
- The standard electrode potential for the elements chlorine and magnesium are:-
Cl2(g) + 2e- → 2Cl-(aq) Eθ =+ 1.36V
Mg2+(aq) + 2e- → Mg(s) Eθ = - 2.36V- Which one of the two elements will act as an oxidizing agent? Explain.
- Calculate the electromotive force of a cell where the overall reaction is:-
Cl2(g) + Mg(s) → MgCl2(s)
- The table below gives the reduction standard electrode potentials for divalent metals.
The letters are not their actual symbols. Use them to answer the questions that follow:-
Metal Eθ (volts) P +1.50 Q - 0.44 R +0.34 S +0.76 - Select two metals whose half cells can produce the highest voltage when connected.
- Draw a well labelled diagram of electrochemical cell formed by half-cells of metals P and Q
- Calculate the voltage produced by the cell in (ii) above
- When nitrate solution of a certain metal X was electrolysed, 1.174g of metal X was deposited by a current of 4 amperes flowing for 16minutes. Determine the formula of the metal nitrate. (1F= 96,500, R.A.M of X= 59)
- The standard electrode potential for the elements chlorine and magnesium are:-
- Study carefully the information given below and answer the questions that follow:-
Substance Physical
state at e.t.pSolubility in
waterOther information A Solid - Soluble
- Blue solution- solution conducts electricity forming two
products B and C
- B is solid and C is a greenish –yellow gasD Gas - Soluble
- Colourless
solution- Solution forms pale blue precipitate with A
and then deep blue solution in excessE Solid - Insoluble - With a solution of A forms B and a
colourless solution at E2+ions- Identify the substances represented by the letters
- Give equations for the reactions in which:-
- Substance B is formed from the solution of A on electrolysis
- Substance B is formed from solution A when reacted with E
- Give one use of gas C
- Name the ion responsible for the deep blue solution
-
- Study the standard electrode potentials for the elements given below and answer the questions that follow. The letters do not represent the actual symbols of the elements
Eθ
Q2(g) + 2e- ⇌ 2Q-(aq) +2.87
R2(g) + 2e- ⇌ 2R-(aq) +1.36
S2+(aq) + 2e- ⇌ S(s) + 1.23
2T+(aq) + 2e- ⇌ T2(g) 0.00
U2+(aq) + 2e- ⇌ U(s) -0.13
V2+(aq) + 2e- ⇌ V(s) -0.76- What is the Eθ value of the weakest reducing agent?
- Which element is likely to be hydrogen? Give a reason for your answer
- Draw a diagram for the cell that would be obtained when the half cell of elements S and V are combined
- Calculate the e.m.f of the electrochemical cell in a (iii) above
- The diagram below represents the electrolysis of dilute sulphuric (VI) acid
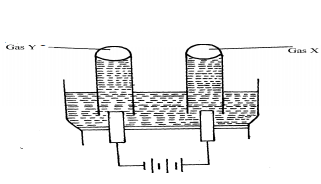
- Name the gases X and Y
- Write ionic equation for the formation of gas X
- At what electrode does reduction take place? Explain your answer
- Name the most suitable electrodes for this experiment. Explain your answer
- Study the standard electrode potentials for the elements given below and answer the questions that follow. The letters do not represent the actual symbols of the elements
- The flow chart below shows an analysis of mixture R that contains two salts. Study it and answer the questions that follow:-
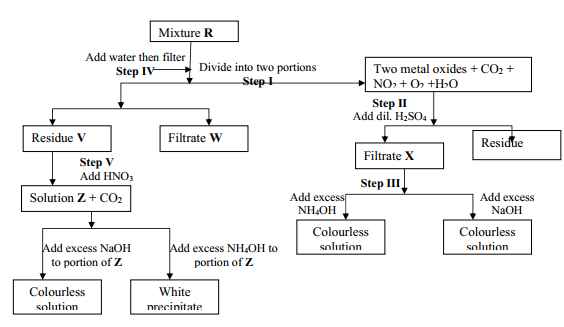
- Write two ionic equations for the reactions between the cation in filtrate X and aqueous ammonia (Ammonium hydroxide)until in excess
- What conclusion can be drawn from Step IV only? Explain
- What observation would indicate the presence of a NO3- ion in step I?
- Write the formula of the anion in residue V. Explain
- Suggest the identity of the cation present in solution Z
- Name the two salts present in mixture R
-
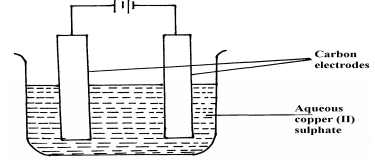
- The set-up below was used in the electrolysis of copper II nitrate solution:
- What is electrolysis?
- Show the anode and cathode on the diagram
- Explain how you would confirm gas P
- Write the equation for the reaction occurring at
Anode ___________
Cathode _________ - State two changes that occur on the electrolyte after the experiment
- Below are the standard electrode potentials for electrodes B and D
B2+(aq) + 2e- ⇌ B(s) Eθ = – 2.92V
D2+(aq) + 2e- ⇌ D(s) Eθ =+ 0.34V- Identify the electrode which is ;
- The least reducing agent
- The strongest oxidizing agent
- Calculate the e.m.f of the cell formed when the two electrodes are connected
- Write a cell representative for the cell above
- Identify the electrode which is ;
- The set-up below was used in the electrolysis of copper II nitrate solution:
- A typical electrolysis cell uses a current of 40,000 amperes. Calculate the mass (in Kg of aluminium produced in one hour). (Al = 27) (Faraday = 96500 Coloumbs )
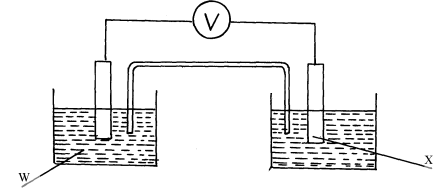
- A strip of copper metal was immersed into a nitrate solution of metal Q overnight. Use the information below to answer questions that follow
information below to answer questions that follow
Eθ (Volts) Q(aq) + e- → Q(s)
Cu2+(aq) + 2e- → Cu(s)+0.80
+ 0.34- State the observations made at the end of the experiment
- Give a reason for the observations made in (a) above
- Calculate the e.m.f of the cell above
-
- Excess marble chips (Calcium carbonate) was put in a beaker containing 150cm3 of dilute hydrochloric acid. The beaker was put on a weighing balance and the total loss in mass recorded after every two minutes as shown in the table below:
Time (min) 0 2 4 6 8 10 Total loss in mass (g) 0 1.8 2.45 2.95 3.2 3.3 - Why was there a loss in mass?
- The average rate of reaction was faster between 0 and 2 minutes than between 6 and 8 minutes. Explain why
- State one way in which the rate of reaction can be increased
- When aqueous sodium sulphate was added to contents of the beaker, a white precipitate was formed;
- Identify the white precipitate ………………………………………………………
- Name one use of the substance named in (iv) (I) above
- A student performed the following experiment with an intention to extract calcium metal
- The student was surprised that no calcium was produced in the experiment. Explain why no calcium was produced
- Write the equation for the reaction that occurred at the anode if the solution was concentrated
- The electrolysis involved passing an electric current of 4A for one hour. Calculate the mass of the product at the anode. (1Faraday = 96500C, Cl =35.5, H = 1.0, O =16, Ca = 40)
- Excess marble chips (Calcium carbonate) was put in a beaker containing 150cm3 of dilute hydrochloric acid. The beaker was put on a weighing balance and the total loss in mass recorded after every two minutes as shown in the table below:
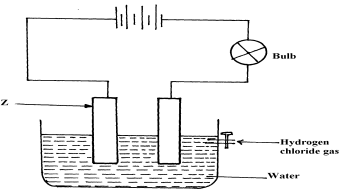
- Cheptoo set-up some apparatus as shown in the diagram below:-
At the start of the experiment, the bulb did not light:-- State and explain the observation made when the tap was opened to allow the hydrogen chloride gas through the water for about 20 minutes
- Write the chemical equation for the reaction that took place at the cathode
- Metals K and N were connected to form a cell as shown in the diagram below. Their reduction potentials are as shown below:
K+(aq) / K(s) Eθ == - 0.17V
N+(aq) / N(s) Eθ == + 1.1 6V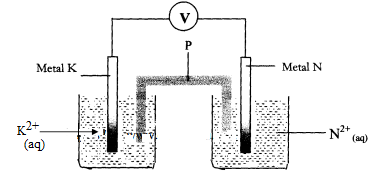
- Write the equation for the half-cell reaction that occurs at
Metal K electrode
Metal N electrode - Identify P and state its role in the above setup
- Identity of P
- Role of P in the setup.
- On the diagram, show the flow of
- Electrons
- Current.
- Calculate cell potential (E) for the cell represented in the setup above
- Write the equation for the half-cell reaction that occurs at
-
- The diagram below shows a Zinc –copper cell.
Given the standard electrode potential of Zinc is -0.76V and that of copper is +0.34V, suggest;- The identity of W …………………………………………………………………....
- The identity of X …………………………………………………………………… .
- The equation for the overall cell reaction
- The reading on the voltmeter
- Sodium hydroxide may be manufactured by the electrolysis of brine as in the diagram below:-
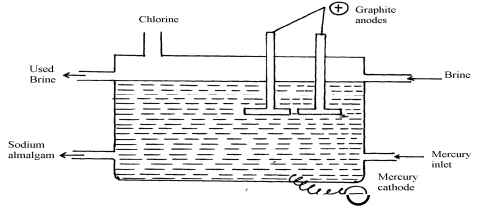
- State the chemical name of brine
- Write the equations for the reactions are the electrodes
Anode……………………………………………………………………
Cathode………………………………………………………………… - Explain how sodium hydroxide is obtained from the product of this process
- The diagram below shows a Zinc –copper cell.
- A typical electrolysis cell uses a current of 40,000 amperes. Calculate the mass (in kilograms) of aluminium produced in one hour (Al=27, 1Faraday = 96,500 coulombs)
- The reaction between ammonia and oxygen to form Nitrogen (II) oxide is highly exothermic
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
The reaction is carried out in presence of platinium-rhodium catalyst at 1173k and a pressure of 911.952k pa.
Explain how each of the following would affect the yield of Nitrogen(II) oxide gas:- Reduction in pressure
- Using a more efficient catalyst
- The following table shows the standard reduction potentials of some half cells. Study the table and refer to it to answer the questions that follow;
Half reaction Eθ volts
P4+(aq) + e- → P3+(aq) +0.61
Q3+(aq) + e- → Q2+(aq) +0.77
R2(g) + 2e- → 2R-(aq) +0.54
S2+(aq) + 2e- → S(s) -0.44
T2+(aq) + 2e- → T(s) -0.74- Identify the strongest oxidizing agent
- Which substance would be used to oxidize R- ion to the atom R
- Study the cell represented below;
T(s)/T2+(aq)//S2+(aq)/S(s)- Identify the electrodes
- Write equations for the reaction taking place in each half- cell
- Determine the cell equation and the electromotive force (e.m.f) of the cell represented in (c) above
- In which direction does the electrons flow in the external circuit of the cell whose e.m.f is determined in (iii) above
- A steady current of 2.5A was passed for 15 minutes through a cell containing divalent ions M2+. During this process 0.74g of metal M was deposited (IF = 96500C)
- Calculate the quantity of electricity passed in this cell
- Determine the relative atomic mass of M
- In the equation below identify the reagent that acts as an acid in the forward reaction. Give a reason for your answer.
NH4+(aq) + H2O(l) ⇌ NH3(aq) + H3O+(aq) - A student set up the experiment shown below. Study it and answer the questions that follow.
- State any two observations the student made during the experiment
- Explain what happens to the pH of the resultant solution at the end of the experiment
- Copper (II) sulphate solution was electrolysed using copper electrode. A Current of 0.5A was passed for 64.3 minutes and a mass of 0.64g of copper was deposited. (Cu = 63.5)
- Which electrode decreased in mass during electrolysis? Explain
- Calculate the quantity of charge needed to deposits 1 mole of copper
- State and explain what is observed when crystals of iodine are heated gently
-
- State Faradays First Law of Electrolysis
- Calculate the volume at s.t.p of hydrogen evolved when 2A of electricity are passed through dilute sulphuric acid for 2hours.
(Molar gas volume at s.t.p = 22.4dm3, one Faraday= 96500coulombs)
- The following is an equation for the reaction between ammonia and water
NH3(g) + H2O(l) ⇌ NH4+(aq) + OH-(aq)
Name the base in the backward reaction - The common ores of Zinc are zinc blende and calamine:-
- Give the chemical formula of Zinc blende
- Explain how the pollution caused by large scale extraction of Zinc can be reduced by having a fertilizer plant close to it
- The oxides of calcium and phosphorous react as shown below:-
6CaO(s) + P4O10(s) → 2Ca3(PO4)2(s)- Give a reason why these substances react and yet both are oxides
- Work out the oxidation state of phosphorous in P4O10
- State one use of Ca3(PO4)2
- The standard hydrogen electrode is used as the reference electrode. Some of the difficulties in using hydrogen gas as an electrode are:
- Hydrogen is a gas at 25oC
- Hydrogen does not conduct electricity
-The half-cell reaction, 2H+(aq) + 2e- ⇌ H2(g) is slow and takes long to reach equilibrium.
Explain how these difficulties are solved in the standard hydrogen electrode - The following are electrode potentials of the half cells
Half cell Eθ volts
M(aq)/M(s) – 0.76
C2+(aq)/C(s) – 0.34
Calculat the potential difference of the following cell.
M(s)/M2+(aq)//C2+(aq)/C(s) -
- Name two types of isotopes of phosphorous
- Explain why phosphorus is stored in water and not in oil like sodium
- Use the cell representation below to answer the questions that follow:-
X(s)/X3+(aq)//W2+(aq)/W(s)- Write the equation for the cell reaction above
- If the e.m.f of the cell is 0.30V and Eθ value for W2+/W is -0.44volts, calculate the Eθ for X3+(aq)/X(s)
- The following diagram represents the electrolysis of dilute sodium chloride solution using inert electrodes
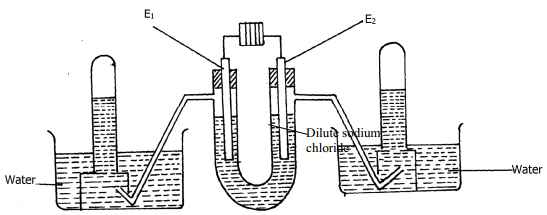
Determine the electrode at which different electrolytic products would be produced if the solution is electrolysed for several hours. Explain - Complete the following redox equations by adding the correct number of electrons on either reactant or product side of the redox equations:-
- ClO3-(aq) + 6H+(aq) → Cl2(g) + 3H2(l)
- NO2-(aq) + H2O(l) → NO3-(aq) + 2H+(aq)
- The following are standard reduction potentials;
Half-cell Eθ/Volts Using iron
Al(s)/Al3+(aq) - 1.66
Zn(s)/Zn2+(aq) - 0.76
Fe(s)/Fe2+(aq) 0.44
Ni(s)/Ni2+(aq) 0.25
Rewrite the Eθ values of the above half-cells using iron as a reference electrode - Calculate the mass of metal J that would be dissolved at the anode when a solution of J (III) nitrite is electrolysed using a current of 1.5amperes for 15minutes (1 Faraday = 96,500C; J = 52)
- Consider the following standard electrode potentials:
Sn2+(aq) + 2e- → Sn(s) Eθ =+0.144v
Fe2+(aq) + 2e- → Fe(s) Eθ = - 0.44v
Zn2+(aq) + 2e- → Zn(s) Eθ = - 0.76v
Some modern cars are made from steel coated with other metals. Using this data above state and explain the best suited metal for coating steel
Answers
-
- Carbon – carbon/ platinum – carbon
- - The concentration of magnesium sulphate increase
- Hydrogen and oxygen given off at the electrodes reduce the water content
- Cu2+(aq) + 2e- → Cu(s)
Mass = RAM × q
f × e
but q = It
1.48 = 63.5 x I x 2.5 x 3600
96500 x 2
I = 1.48 x 2 x 96500
63.5 x 2.5 x 3600
= 0.5 A -
- Anode is electrode A (1 mk)
B is cathode - 2H+(aq) + 2e- → H2 (g)
- The acid becomes more
- Anode is electrode A (1 mk)
-
- 200 × 58 × 60 C → 64.8g ✓ ½
9500C → 27g ✓ ½
27 ×200 × 58 × 60 ✓ ½ = +3 ✓ ½
64.8 × 96500 - 40H-(g) → 2H2O(l) + O2(g) +4e- ✓ ½
4 × 96500 → 22.4dm3 ✓ ½
200 × 58 × 60 × 22.4
4 × 96500 C
= 40.39 dm3 ✓ ½
- 200 × 58 × 60 C → 64.8g ✓ ½
-
- Mg(s) + Pb2+(aq) → Mg2+ (aq) + Pb(s)
- 0.13 – (-0.76)
= +0.53V
-
- 2F = 10 ⇒ 2F – 10 = 0; 2F = 10 ∴F = +5
F = +5 (penalize -5) - Group V
- 2F = 10 ⇒ 2F – 10 = 0; 2F = 10 ∴F = +5
- Aluminium has a higher electrical conductivity than sodium. √1 Aluminium has three delocalized √½ electrons in its metallic structure while sodium has only one delocalized electron in its structure. √½
- Q = It √½
= 3 x 50 x 60 √½
= 9000 C √½
1 mole of Zn is liberated by a charge of 2 f.
i.e 96500 x 2 → 65g of Zn
9000C ?
= 65 x 9000 √1 = 12.124g Zn √½
96500 x 2 -
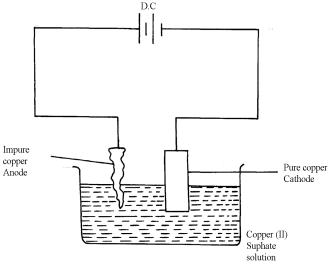
- Q is sulphur (IV) oxide SO2(g). √1
- Impure copper is the while pure copper is cathode. During electrolysis impure copper is purified and pure copper deposited on the cathode as shown in the half electrode reaction below;
CATHODE EQUATION:
Cu2+ + 2e- → Cu(s) √½
- The cathode is therefore removed and replaced after an interval.
-
-
- the yield of NH3 would be lowered √ ½ any supply of heat makes NH3 to decompose to N2 and H2
- the yield of NH3 would be increased
- a catalyst accelerate the rates of both forward and reverse reactions equally√½ . Equilibrium position is not affected by a catalyst√½
-
-
-
- T√
- Z(s) + 2G+ → 2G(s) + Z2+(aq)√1
- Eθ cell = E - E
= 0.08 – (-2.38)√1
= + 3.18
- Mass of due to C = 12/44 x 4.2 = 1.145√ ½
Mass of due to H = 2/18 x 1.71 = 1.889√ ½
Moles of C = 1.145/12 = 0.095√ ½
Moles of H = 0.1889/1 =0.1889√ ½
Moles ratio c : r
0.095 : 0.1889√ ½
1 : 2
E.F = CH2√ ½ (accept alternative method) - 96,500 coulombs = 1 faraday
144,750 coulombs = ?
144,750 faraday√ ½
96,000
= 1.5 faradays√ ½
Copper (II) ions = 2 faradays (penalize ½ mk for missing/wrong units)
2 faradays yield = 64g of copper
1.5 faradays yield = ?
= 1.5 x 64g√ ½
2
=48g of copper was obtained√ ½ - Physical difference:-
Na2O2 is yellow while Na2O is white
Chemical difference:-
N2O2 reacts with water to form NaOH and O2 while
Na2O reacts with water to form NaOH only -
- Pb(NO3)2
- Mg(s)/ Mg2+(aq) // Pb2+(aq)/Pb(s)
-
- MnO4 is reduced;
Oxidation number of Mn is reduced from +7 to +2 - 5Fe2+(g) → 5Fe3+(aq) + 5e-;
- MnO4 is reduced;
-
- 2 Cr(s) → 2Cr3+(aq) +6e-
3Fe2+(aq) + 6e- → 3Fe(g)
2Cr(g) + 3Fe2+(aq) → 2 Cr3+(aq) +3Fe(g) √ - 0.30 = - 0.44 - EθR
EθR = - 0.44 – 0.30
= - 0.74V √
- 2 Cr(s) → 2Cr3+(aq) +6e-
-
- – Filtration of air/electrostatic precipitation/purification
- Passing through sodium hydroxide/potassium hydroxide to absorb Carbon (IV) oxide gas
- Cool to remove water vapour as ice
-Cool remaining air to liquid by repeated compression and expansion of liquid air
- Fractional distillation of liquid air- Nitrogen collected at -196oC -
- Nitrogen (II) Oxide
-
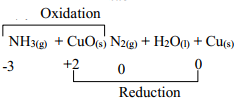
OR - Oxidation number of N2 in NH3 increases from -3 to 0. Oxidation number of reducing agent increases or oxidation number of Cu in CuO decreases from +2 to 0 hence is a reducing agent - NH4NO3 → N2O + 2H2O
- Fertilizer/expose
-
- G or G
- E2+(aq) + 2OH-(aq) → E(OH)2(s)
- – Filtration of air/electrostatic precipitation/purification
-
- G//G2(g) Not G-
It has the highest potential OR highest reduction potential ✓ 1 mark - G and N or G2(g)//N(g) ✓ 1 mark
-
- G//G2(g) Not G-
-
-
- Cathode – steel
Anode – Carbon / graphite - To lower the melting P+ hence reducing cost of heating the salt.
- To prevent the two products from recombining.
- Cathode
Na+(l) + e- → Na(l)
Anode
2Cl-(l) → Cl2(g) + 2e- - less dense than electrolyte/ has low density
- Cathode – steel
-
- quantity = 6.42 × 10 × 60 = 3852
- 3852c produces 2.74
2 × 96000 will produce ?
(2 × 96000) × 2.74
3852
= 136.58
-
-
-
- H+(aq) + e- → ½H2
- E cell = 0.76 + 0.54 = +1.3 volts
-
- Fe3+
- Zn
- Fe3+ ion
2Fe3+ + 2e- → 2 Fe2+ Eθ = + 0.77
2I- → I2(g) + 2e- Eθ = - 0.54
2 Fe3+ + 2I- → 2Fe2+ + I2 Eθ = + 0.23
-
-
-
- Chlorine Has a higher reduction potential
- +1.36 2.36 = +3.72
-
- P and S
- +1.50 – 0.44 + + 1.94
- Q = 4 ×6 × 60 = 3840C
1.17g → 3840
59 g → 59 × 3840 = 192981.261 C
1.174
If 96,500c = IF
192891.261 = ?
192981.261 × 1
96500
Charge of X = +2
Formula X(NO3)2
-
-
- B – Copper metal
C – Chlorine gas
D – Ammmonia gas
E – Zinc -
- Cu2+(aq) + 2e- → Cu(s)
- CuSO4 + Zn(s) → ZNSO4 + Cu(s)
Cu2+ + Zn(s) →Cu(s) + Zn2+(aq)
- – Water treatment
-Manufacture of hydrochloric acid - Tetra mine copper (II) ions
- B – Copper metal
-
-
- Eθ = 1.13V
- T2 because it’s standard electrode potential is zero. i.e. point of reference.
-
- E.m.f = + 1.23 - - 0.76 = 1.99 V
-
- x - Oxygen
y – Hydrogen - 4OH-(aq) → 2H2O + O2 + 4e-
- Reduction takes place at electrode Y. H+ ions gain electrons to form hydrogen gas.
- Platinium / graphite/ Nickel because it is inert.
- x - Oxygen
-
-
- Zn2+(aq) + 2OH-(aq) → Zn(OH)2(s)
Zn(OH)2(s) + 4NH3(aq) → Zn(NH3)42+(aq) + 2OH-(aq) - The mixture consists of a soluble compound and an insoluble compound.
- Evolution brown fumes of NO2 gas
- CO32- - Because its reaction with HNO3 produces CO2 gas or 2H+(aq) + CO32-(aq) + H2O(l) +CO2(g)
- Pb2+ ion
- Lead (ii) Carbonate
Zinc (II) Nitrate
- Zn2+(aq) + 2OH-(aq) → Zn(OH)2(s)
-
-
- Process by which an electrolyte is decomposed by passing an electric current through it.
- Anode – left pt rod
Cathode – right pt rod - – Blue /pale green colour fades
- P solution becomes acidic
-
-
- – D2+
- – D2+
- C
E cell = Eordn – Eordn
= +0.34 –(-2.92) = +3.26V - B(s)/B2+(aq)//D2+(aq)/D(s); E = + 3.26V
-
-
- Q = 40000 x 60 x 60 = 144000000c
Mass of Al = 144000000 x 27
3 x 96500
= 13.43kg -
- Strip of copper metal dissolved forming blue solution. √½
- Copper displaces ions √½ of Q from solution since copper is more electropositive √½ than Q.
- E.m.f of cell = (0.80 - 0.34)V √½
= 0.46V √½
-
-
- Carbon (IV) Oxide gas evolved was lost to the atmosphere
- Concentration of reactants higher between O and R
Reaction rate faster - Grinding the marble chips
- Calcium sulphate
- Plaster of Paris
-
- Hydrogen ions discharged;
It takes less energy than calcium ions - 2Cl-(aq) → Cl2(g) + 2e-
- Q = 1t = 4 x 1 60 x 60 ( ½ mk)
= 14400C
2 x 96500C = 2 x 35.5 (½mk)
14400C = ?
14400 x 2 x 35.5
2 x 95600
= 5.297g (½mk)
- Hydrogen ions discharged;
-
-
- the bulb light√ ½
Hydrogen chloride gas ionized in water to give H+ and cl-(aq) that are responsible for conduction of electric current√1 - 2H+(aq) +ze- H2(g)√1
- the bulb light√ ½
-
- K(s) → K2+(aq) + 2e-
N(s) + 2e- → N(g) -
- Salt bridge
- - Complete the circuit
- Balance the ions in each half cell
- E cell = E Red – E oxd
= +1.16 – (-0.17) = +1.33V
- K(s) → K2+(aq) + 2e-
-
-
- Zinc sulphate / Zinc chloride / Zinc nitrate solution
- Copper
- Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
- E = 0.34 + 0.76
= 1.0V
-
- Concentrated sodium chloride solution
- 2 Cl-(aq) → Cl2(g) + 2e-
Na+(aq) + e- → N(l) - Sodium amalgam is flown into water. It reacts forming sodium hydroxide solution
-
- Quantity of electricity = (40,000 x 60 x 60) Coulumbus ✓ ½ mark
3 x 96,500 Coulumbus produce 27g of Al
჻ 40,000 x 60 x 60 will produce
40,000 x 60 x 60 x 27 Kg ✓ ½ mark
3 x 96,500 x 1000 ✓ ½ mark
= 13.43Kg ✓ ½ mark
Subtract ½ mark if units missing or wrong
[Total 12 marks] -
- Increased yield of NO/✓1 mark Equilibrium shifts to the right // favours the forward reaction// reduced pressure favours forward reaction// increased volume number of molecules
- It will not affect the yield // remains the same
Catalyst do not affect position of Equilibrium
-
- R
- T
-
- T(g) and S(g)
- Half cell one Half cell two
T(s) − 2e- → T2+ S2+(aq) + 2e → S(s)
OR: T(s) → T2+(aq) + 2e- - T(s) → T2+(aq) + 2e-, E = +0.74V
- From T(s)/T2+ half cell to S2+/S(s) half cell through conducting wires
-
- Q = It
= 2.5 x (15x60)
= 2250C - RAM = mass x valency x 96500
Q
= 0.74 x 2 x 96500
2250
= 142820/2250
= 63.476
- Q = It
- NH4+√ 1, proton donor√
-
- - Bubbles of colourless gas at the anode√ ½
- Brown deposits at the cathode √ ½
- Blue color of the solution fades Any 2 ½ mark each - The Ph decreases
Removal of OH- ions leaves an excess of H+ hence the solution becomes more acidic√
- - Bubbles of colourless gas at the anode√ ½
-
- Anode. Copper anode dissolves
- Q = 0.5 X 60 X64.3 = 1929C
0.64g of Cu → 1929 C
჻ 63.5 of Cu → ?
63.5 X 1929√ ½
0.64
= 191393 C √ ½
- The grey-black solid changes to purple gas iodine sublimes at low temperature due to weak Van der walls forces
-
- The mass of substance liberated during electrolysis is directly proportional to the quantity of electricity passed
- Quantity of electricity = 2 x 2 x 36000 = 14400c (½mk)
Volume of gas evolved = 14400 x 22.4 = 1.671dm3 (1 ½ mk
2 x 96500
- OH- √1 (1 mk)
-
- ZnS - No mark if the letters are not joined
- SO2 produced as a by-product is used in contact process to obtain H2SO4. This acid is used in making fertilizers e.g. ammonium sulphate
-
- CaO is basic and P4O10 is acidic
- Let the ON of P be x
4x + (-2 x10) = 0
4x/4 = +20/4
x = +5 - Used as a fertilizer
-
- Platinum electrode is used, H2 is bubbled over the pt electrode immersed in 1M H+ i.e 1M HCl.
The electrode is coated with finely –divided platinum catalyst - electrochemical cell
- Platinum electrode is used, H2 is bubbled over the pt electrode immersed in 1M H+ i.e 1M HCl.
- + 0.76 + 0.34 = 1.0Volts
-
- - Red- Phosphorous
- White – Phosphorous - Phosphorous is insoluble in water because its non-polar while water is polar.
It cannot be stored in oil because oil is non-polar it will dissolve the phosphorous.
- - Red- Phosphorous
-
- 2X(s) + 3W(aq) → 2X3+(aq) + 3W(s)
- Eθ(X/X3+(aq) + - 0.44 = 0.3V
Eθ(X(s)/X3+(aq) = +0.74V
Eθ(X3+(aq)/X(s) = -0.74V
- Electrode - E1 is the anode
Dilute electrolyte – OH- ions are discharged.
4 OH-(aq) → 2H2O(l) + O2(g) + 4e-
Oxygen gas is produced.
Discharge of hydroxyl ion increases the concentration of sodium chloride.
Chloride, Cl-, are then discharged
Chloride gas is produce
2Cl-(aq) → Cl2(g) + 2e- -
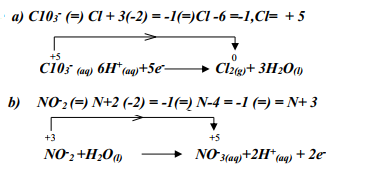
-
Half Cell Eθ/V Eθ/V using iron ref - electrode Al(s)/Al3+(aq) - 1.66 - 1.22 Zn(s)/Zn2+(aq) - 0.76 +0.32 Fe(s)/Fe2+(aq) - 0.44 0.00 Ni(s) /Ni2+(aq) - 0.25 + 0.19 - θ = 1.5 X 60 X 15 = 1350
J3+(aq) + 3e- → J(s)
3F = 3 × 96500 = 289 500C
289500C deposit = 52g of J(s)
1350 C deposit = ?
1350 × 52
289500
= 0.2425g - Tin (Sn) its oxidation potential is +0.144V. It is the least likely to combine/ react with elements of weather
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Electrochemistry Questions and Answers - Chemistry Form 4 Topical Revision.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students