- The grid below shows part of the periodic table. Use it to answer the questions that follow. The letters do not represent actual symbols.
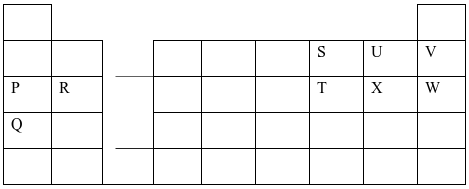
- Which of the elements has the highest atomic radius? Explain. (2 marks)
Q- This is because it has the highest number of energy levels. - Identify the most reactive Oxidizing agent. Explain. (2 marks)
U – This is because U has the highest nuclear change due to its small atomic radius among the non metals. - Compare the atomic radius of P and R. Explain (2 marks)
P has bigger atomic radius than R. This is because P has higher nuclear charge than R. - Give the formula of one stable ion with an electron arrangement of 2.8 which is:
- A Negatively charged divalent ion. (2marks)
S2- -
A Positively charged monovalent
P+
- A Negatively charged divalent ion. (2marks)
- Given that the mass number of W is 40. Write down the composition of its nucleus.(2 marks)
P = 18 N = 40 – 18 = 28
P = 18
N = 22 - Write the formula of the compounds formed between.
- Element R and X. (1 mark)
RX2 - Give one property of the structure formed when R and X bond. (1 mark)
Compounds with the above structures are soluble in water but insoluble in organic conduct.
Compounds with the above structure conduct electricity in molten and aqueous state but they are non conductors in solid state.
Compounds with the above structure exist in crystalline form
Compounds with the above structure have very high melting and boiling point.
- Element R and X. (1 mark)
- Which of the elements has the highest atomic radius? Explain. (2 marks)
- An aqueous solution of zinc sulphate is electrolysed using platinum electrodes as shown in the set up below.
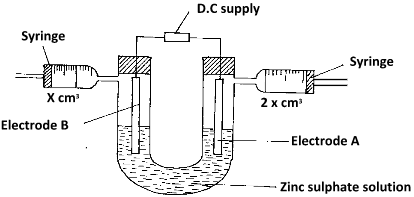
- Write a half equation for the reaction taking place at electrode A. (1mark)
2 H +(aq)+ 2e - H2(g) - Identify electrodes A and B (2 marks)
A-Anode B-cathode - State and explain the observation at electrode B if copper plate was used instead of platinum electrode. (2marks)
B reduces in size;copper anode dissolves Cu(s) Cu 2+(aq) + 2e-
- Write a half equation for the reaction taking place at electrode A. (1mark)
- 0.22g of metal Q is deposited by electrolysis when a current of 0.06A flows for 99 minutes.
(RAM of Q = 184, 1F = 96500c)- Find the number of moles of Q deposited. (1 mark)
Moles of G = 0.22 = 0.0012 mole
184 - Determine the value of n in the metallic ion Qn+ (3 marks)
Q = IT =0.06 x 99 x 60 = 356.4C
0.0012mole 356.4C
1mole ?
1 x 356.4
--------------- = 297000C
0.0012
1mole of – e = 96500C
? = 297000C
297000 x1
------------------- =3.078
96500
n = +3
- Find the number of moles of Q deposited. (1 mark)
- Determine oxidation number of chlorine in ClO3- (1mark)
x + 3 (-2) =1
x + -6 -1
x = -1+6
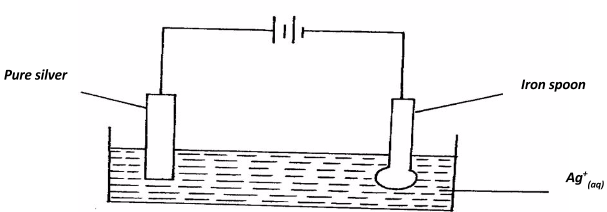
x = +5 - An iron spoon is to be electroplated with silver. Draw a labelled diagram to represent the set-up that could be used to carry out this process. (2marks)
- The scheme below was used to prepare a cleansing agent. Study it and answer the questions that follow.
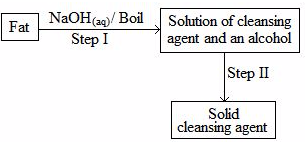
- What name is given to the type of cleansing agent prepared by the method above? (1mark)
Soap. - Name one chemical substance added in step II (1mark)
Concentrated NaCl/ Brine/ NaCl(l) - What is the purpose of adding the chemical substance named in a (ii) above? (1mark)
To precipitate out the soap - Name any other suitable substance that can be used in step I (1mark)
potassium hydroxide/ KOH(aq) - Explain how an aqueous solution of the cleansing agent removes oil during washing (2marks)
– Cleansing agent is made up of non- polar (hydrocarbon) and polar (head)
-When mixed with oil /grease, the hydrocarbon part is attracted to it. while the polar part stays in water
-The oil particles are broken and carried off to the solution.
- What name is given to the type of cleansing agent prepared by the method above? (1mark)
- Study the scheme below and answer the questions that follow.
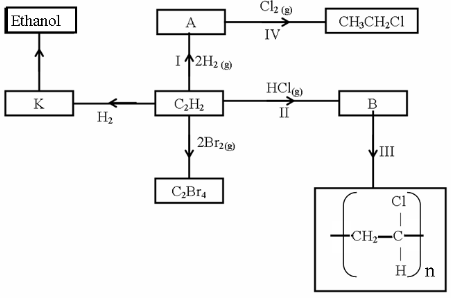
- Identify the catalyst used in step I (1mark)
Uv light - Name the compounds A and B (2 marks)
A ethane B 1-chloroethane - Give one disadvantage of compound formed in step III (1mark)
It is non biodegradable hence causes environmental pollution.any one - Name the reactions taking place at steps: (1mark)
I hydrogenation IV halogenation - Describe how substance K is converted to ethanol (2marks)
-add concentrated sulphuric (vi) acid to ethane to form ethylhydrogensuplphate
C2H4(g) + H2SO4(l) -------------------> C2H5OSO3H(aq)
-add water to the mixture.ethylhydrogensulphate is hydrolysed to ethanol
CH3CH2OSO3H(aq) +H2O(l) ------------> CH3CH2OH(aq) + H2SO4(aq)
- Identify the catalyst used in step I (1mark)
- The scheme below was used to prepare a cleansing agent. Study it and answer the questions that follow.
- State the Hess’ law. (1 mark)
The overall heat change of a reaction is the same regardless of the route through which the reaction occurs. - The enthalpies of combustion of calcium, carbon and decomposition of calcium carbonate are indicated below;
C a (s) + ½O2 (g)--------> CaO (s): ΔH=-635 kJmol-1
C (s) + O2 (g) -------------> CO2 (g) ΔH=-394 kJmol-1
Enthalpy of decomposition of CaCO3 = +178 kJmol-1- Draw an energy cycle diagram that links the enthalpy of formation of calcium carbonate to enthalpies of combustion of calcium, carbon and decomposition of calcium carbonate.
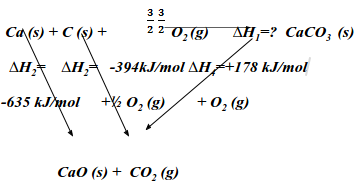
- Determine the enthalpy of formation of calcium carbonate. (2 marks)
∆H1= ∆H2 +∆H3 + ∆H4 (1mk)
= -635 + (-394) - (+178)
= -1207 kJ/mol
- Draw an energy cycle diagram that links the enthalpy of formation of calcium carbonate to enthalpies of combustion of calcium, carbon and decomposition of calcium carbonate.
- Some average bond energies are given below.
Bond Energy in kJ/mol C-C 348 C-H 414 Cl-Cl
243
C-Cl
432
H-Cl
340
Calculate the energy change for the reaction below. (3 mks)
C2H6 (g) + Cl2 (g) CH3CH2Cl(g) + HCl(g)
- State the Hess’ law. (1 mark)
- Define the term solubility. (1 mark)
Solubility is the maximum mass of solute that dissolves in 100 g of a solvent at a particular temperature. - In an experiment to determine the solubilities of two salts X and Y at different temperatures, a candidate recorded her observations as shown below.
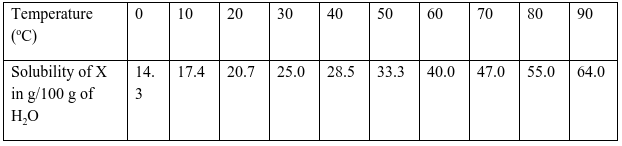
On the same axes plot the solubility curves of X and Y. (4 marks)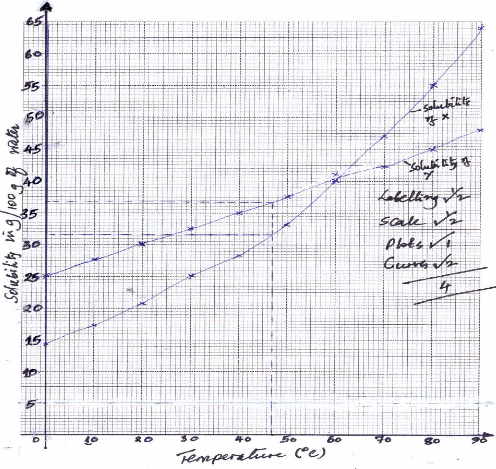
- From your graph to determine;
- The solubility of X and Y at 47 oC
Solubility of X (1 mark)
X:31.5 (±0.2) g/100g of water
Solubility of Y (1 mark)
Y: 36.6 (± 0.2 g/100g of water - The temperature at which the two salts are soluble in water.(1 mark)
60.5 ±1 oC
- The solubility of X and Y at 47 oC
- If 60g of X is dissolved in 100 g of water and heated to 90oC, calculate the amount of salt that crystallized out if cooled to 20 oC.(1 mark)
60-20.7 =39.3 g - State what would happen if a mixture of 55g of salt X in 100 g of water and 30 g of Y in 100 g of water were cooled from 90 oC to 70 oC.(3 marks)
13 g of X will crystallize, salt Y will be in solution . The solubility of X is lower than the mass dissolved while that of N is higher than the mass dissolved. - State one application of solubility.(1 mark)
Fractional crystallization (any one)
- Define the term solubility. (1 mark)
- The flow chart below shows some processes in the extraction of zinc. Study it and answer the questions that follow.
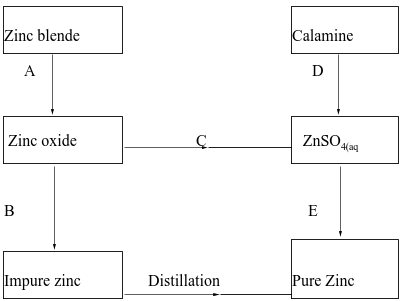
- Name the processes represented by A and E. (2 marks)
A - Roasting
E - Electrolysis - State the reagents required for processes B, C and D. (3marks)
B Carbon
C dil. Sulphuric acid
D dil. Sulphuric acid - Write a chemical equation of the reaction that occurs in process B. (1mark)
ZnO(s) + C (s )----------> Zn(s) + CO (g) - With an aid of a diagram, explain how you would obtain a pure sample of zinc by process E
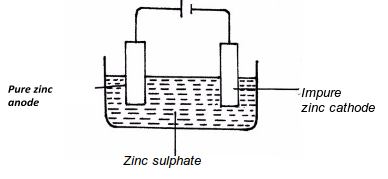
- State two commercial uses of zinc metal. (1 mark)
- Electroplating of iron sheets any two 1mk
- Zinc case for dry cells
- Making alloys e.g. brass
- Name the processes represented by A and E. (2 marks)
- The diagram below shows a set-up of apparatus that can be used to prepare nitrogen (IV) oxide. Study it and use it to answer the questions that follow
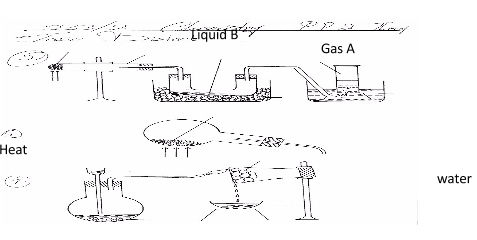
- Write the equation for the reaction that takes place in the boiling tube. (1 mark)
2Pb(NO3)2(s)---------> 2PbO(s) + 4NO2 (g)+ O2(g) - State the observations made in the boiling tube. (2 marks)
- brown fumes of NO2
-solid residue is red when hot but on cooling it becomes yellow - Explain why lead (II) nitrate is preferred over other metal nitrates in this experiment.
Lead (II) nitrate crystalises without water of crystalisation that would interfere with the NO2 formed - Describe how gas A can be identified. (1 mark)
By using a glowing splint. Gas A relights a glowing splint
- Write the equation for the reaction that takes place in the boiling tube. (1 mark)
- Name liquid B (1 mark)
dinitrogentetraoxide - Write a chemical equation to show how liquid B is formed in this experiment. (1mark)
2 NO2(q) --------cool-------> N2 O4(l)
- Name liquid B (1 mark)
- In another experiment, excess aqueous lead (II) nitrate solution was reacted with a solution which contained 2.34g of sodium chloride. Calculate the mass of precipitate formed in this reaction. (3 marks)
(Pb = 207, Cl = 35.5, Na = 23)
P b(NO3)2 (aq) + 2NaCl(aq)------------> PbCl2(s) + 2NaNO3(aq)
1mol 1mol
117g 278 g
2.34g x
x = 2.34 x 378
----------------
117
= 5.56g of PbCl2 - Write an ionic equation for the reaction that takes place when nitrogen (IV) oxide reacts with aqueous sodium hydroxide. (1 mark)
2 OH-(aq) + Cl2(g) -------------------> Cl-(aq) +OCl-(aq) + H2 O(l)
- In another experiment, excess aqueous lead (II) nitrate solution was reacted with a solution which contained 2.34g of sodium chloride. Calculate the mass of precipitate formed in this reaction. (3 marks)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download KASSU JOINT EVALUATION EXAMINATION CHEMISTRY Paper 2 with answers.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

