- Two reagents that can be used to prepare chlorine gas are manganese (IV) oxide and concentrated hydrochloric acid.
- Write an equation for the reaction. (1 mark)
- Give the formula of another reagent that can be reacted with concentrated hydrochloric acid to produce chlorine gas. (1 mark)
- Describe how the chlorine gas could be dried in the laboratory. (2 marks)
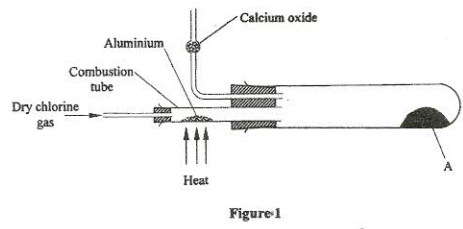
- In an experiment, dry chlorine gas was reacted with aluminium as shown in figure 1.
- Name substance A. (1 mark)
- Write an equation for the reaction that took place in the combustion tube. (1 mark)
- 0.84 g of aluminium reacted completely with chlorine gas. Calculate the volume of chlorine gas used. (Molar gas volume is 24dm3, Al = 27) (3 marks)
- Give two reasons why calcium oxide is used in the set up. (2 marks)
- Two reagents that can be used to prepare chlorine gas are manganese (IV) oxide and concentrated hydrochloric acid.
- Draw the structures of the following compounds: (2 marks)
- 2- methylbut -2 –ene;
- heptanoic acid.
- Describe the physical test that can be used to distinguish between methanol and hexanol. (2 marks)
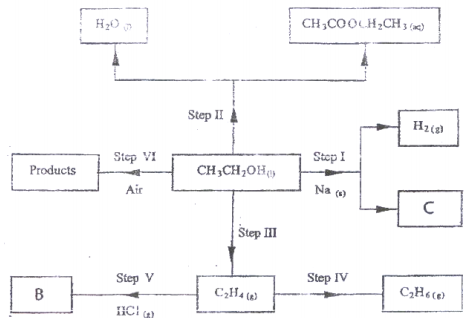
- Use the flow chart below to answer the questions that follow.
- Name:
- the type of reaction that occurs in step II; (1 mark)
- Substance B. (1 mark)
- Give the formula of substance C. (1 mark)
- Give the reagent and the conditions necessary for the reaction in step (IV). (4 marks)
- Name:
- Draw the structures of the following compounds: (2 marks)
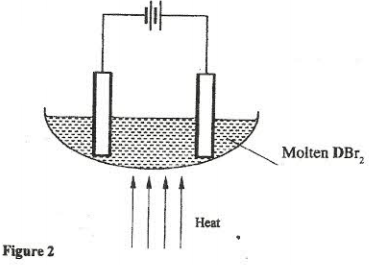
- The set-up below. (figure 2) was used to electrolyse a bromide of metal D, DBr2.
- Write the equation for the reactions at the:
- cathode. (1 mark)
- anode (1 mark)
- The electrodes used in the experiment were made of carbon and metal D.
Which of the two electrodes was used as the anode? Give a reason. (2 marks) - Give a reason why this experiment was carried out in a fume cupboard. (1 mark)
- When a current of 0.4A was passed for 90 minutes, 2.31 g of metal D were deposited.
- Describe how the amount of metal D deposited was determined. (3 marks)
- Calculate the relative atomic mass of metal D. (1 Faraday = 96,500 coulombs) (3 marks)
- Write the equation for the reactions at the:
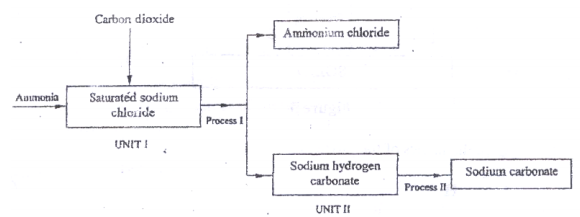
- The schematic diagram shows part of the solvay process used for the manufacture of sodium carbonate.
- Explain how the sodium chloride required for this process is obtained from sea water. (2 marks)
- Two main reactions take place in UNIT 1. The first one is the formation of ammonium hydrogen carbonate.
- Write the equation for this reaction. (1 mark)
- Write an equation for the second reaction. (1 mark)
- State how the following are carried out: (2 marks)
- Process 1
- Process II
- In an experiment to determine the percentage purity of the sample of sodium carbonate produced in Solvay process, 2.15 g of the sample reacted completely with 40.0cm3 of 0.5 M sulphuric acid.
- Calculate the number of moles of sodium carbonate that reacted. (2 marks)
- Determine the percentage of sodium carbonate in the sample.
(Na = 23.0, C = 12.0, O = 16.0) (2 marks)
- Name two industrial uses of sodium carbonate . (2 marks)
- The schematic diagram shows part of the solvay process used for the manufacture of sodium carbonate.
-
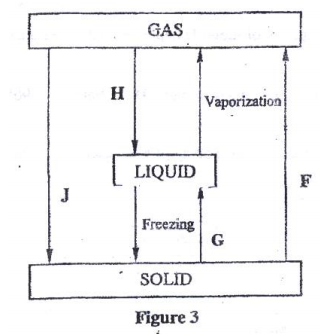
- Figure 3 shows the changes that take place between states of matter. Some of them have been identified and others labelled.
- Give the names of processes:
- H (1 mark)
- G (1 mark)
- Name one substance that can undergo process F when left in an open container in the laboratory. (1 mark)
- The process J is called deposition. Using water as am example, write an equation to represent the process of deposition. (1 mark)
- Give the names of processes:
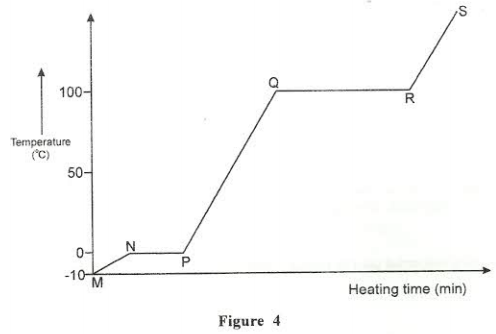
- Figure 4 shows the heating curve of water.
- Give the names of the intermolecular forces of attraction in the segments;
- MS (1 mark)
- RS (1 mark)
- The heats of fusion and vaporization of water are 333.4 Jg-1 and 1159.4 Jg-1 respectively.
- Explain why there is a big difference between the two. (2 marks)
- How is the difference reflected in the curve? (1 mark)
- Give the names of the intermolecular forces of attraction in the segments;
- Coal, oil and natural gas are major sources of energy. They are known as fossil fuels. Hydrogen is also a source of energy.
- State and explain two reasons why hydrogen is a very attractive fuel compare to fossils. (3 marks)
- State one disadvantage of using hydrogen fuel instead of fossil fuels. (1 mark)
- Figure 3 shows the changes that take place between states of matter. Some of them have been identified and others labelled.
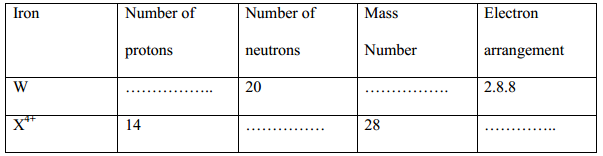
- Study the table below and complete it. (W- and X4+ are not the actual symbols of the ions). (2 marks)
- State the observation that would be made in the following tests to distinguish between:
- Sodium and copper burning pieces of each in air. (2 marks)
- Sodium and magnesium by placing small pieces of each in cold water which contains two drops of phenopthalein. (2 marks)
- The atomic number of Na and Mg are 11 and 12 respectively. Which of the elements has a higher ionization energy? Explain. (2 marks)
- Naturally occuring uranium consists of three isotopes which are radioactive.
Isotope 234U 235U 238U Abundance 0.01% 0.72% 99.27% - Which of these isotopes has the longest half-life? Give a reason. (1 mark)
- Calculate the relative atomic mass of uranium.
- 23592U is an alpha emitter. If the product of decay of this nuclide is thorium (Th). Write a nuclear equation for the process. (1 mark)
- State one use of radioactive isotopes in the paper industry.
- Study the table below and complete it. (W- and X4+ are not the actual symbols of the ions). (2 marks)
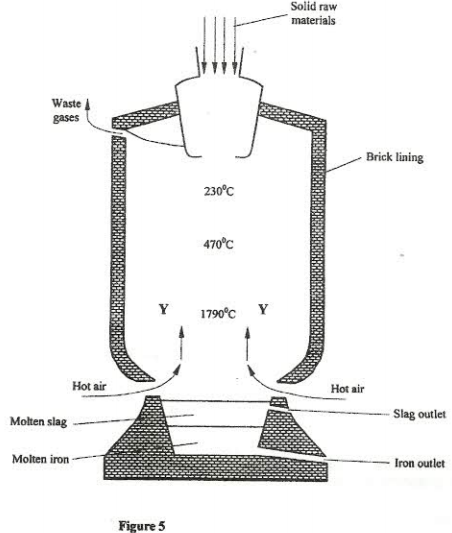
- Iron is obtained from haematite using a blast furnace shown in the figure 5 below.
- Four raw materials are required for the production of iron. Three of these are iron oxide, hot air and limestone.
Give the name of the fourth raw material. (1 mark) - Write an equation for the reaction in which carbon (IV) oxide is converted into carbon (II) oxide. (1 mark)
- Explain why the temperature in the region marked Y is higher than that of the incoming hot air. (2 mark)
- State the physical property of molten slag other than density that allows it to be separated from molten iron as shown in figure 5. (1 mark)
- One of the components of the waste gases in Nitrogen (IV) oxide.
Describe the adverse effects it has on the environment. (2 marks) - Iron from the blast furnace contains about 5% carbon.
- Describe how the carbon content is reduced. (2 marks)
- Why is it necessary to reduce the carbon content? (1 mark)
- Four raw materials are required for the production of iron. Three of these are iron oxide, hot air and limestone.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Kenya Certificate Of Secondary Education (KCSE 2009) Chemistry Paper 2.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students