QUESTIONS
- You are provided with:
- solution J containing copper (II) ions
- solution K, 0.1 M sodium thiosulphate
- aqueous potassium iodide, solution L
- starch indicator, solution M
You are required to determine the: - concentration of copper (II) ions in solution J
- enthalpy change of reaction between copper (II) ions and hydroxide ions.
PROCEDURE I
- Using a pipette and a pipette filler, place 25.0 cm3 of solution J in a 250 ml volumetric flask. Add distilled water to make upto the mark. Label this as solution J2. Retain solution J for use in procedure II.
- Place soluton K in a burette. Using a clean pipette and pipette filler, place 25.0 cm3 of solution J2 in a 250 ml conical flask. Add 10cm3 of potassium iodide, solution L. Shake well, then add 2 cm3 of starch indicator, solution M. Titrate until a blue-black colour appears and continue titrating until the blue-black colour just disappears.
Record you readings in Table 1 below. - Repeat step (b) two more times and complete Table 1. (3 marks)
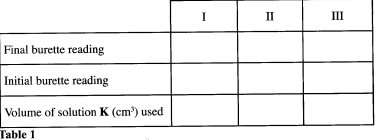
Calculate the:- average volume of solution K used. (1 mark)
- moles of sodium thiosulphate used; (2 mark)
- concentration in moles per litre of copper (II) ions in solution J given that the number of moles of copper (II) ions in 25.0 cm3 of solution J2 are the same as the moles of sodium thiosulphate used. (2 1/2 marks)
PROCEDURE II
- Using a clean burette, place 5.0 cm3 of solution N into each of the six (6) test-tubes.
- Using an 100 ml measuring cylinder, place 20 cm3 of solution J in a 100 ml plastic beaker. Measure the temperature of solution J and record in Table 2 below.
- To solution J in the beaker, add sodium hydroxide, solution N from one of the test-tubes. Stir the mixture with the thermometer and record in Table 2, the maximum temperature reached. Continue with step (d) IMMEDIATELY.
- Add the sodium hydroxide, solution N from another test-tube to the mixture obtained in (c) above, stir and record the maximum temperature reached in Table 2. Continue adding the sodium hydroxide, solution N from each of the other four test-tubes, stirring the mixture and recording the maximum temperature each time and complete Table 2.
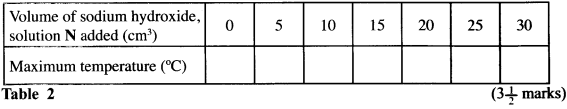
- On the grid provided, plot a graph of temperature (vertical axis) against volume of sodium hydroxide solution N added. (3 marks)
- Using the graph, determine the:
- volume of sodium hydroxide, solution N that reacted completely with 20 cm3 of solution J; (2 marks)
- temperature change, ΔT, for the reaction: (1 mark)
- Enthalpy of the reaction per mole of copper (II) ions. (Heat capacity = 4.2 J g-1k-1, density of the mixture = 1.0 gcm-3) (3 marks)
- On the grid provided, plot a graph of temperature (vertical axis) against volume of sodium hydroxide solution N added. (3 marks)
- You are provided with substance P. Carry out the tests below and write your observations and inferences in the spaces provided.
- Describe the appearance of substance P. (1 mark)
- Place about one-third of substance P in a dry test-tube and heat it strongly.
observations(1 mk) | Inferences(1mk) - Place the remaining amount of substance P in a boiling tube. Add about 10 cm3 of distilled water and shake well. Retain the mixture for tests in (d) below.
observations(1 mk) | Inferences(1mk) - Use about 2 cm3 portions of the mixture obtained in (c) for tests (i) to (iii) below.
- Add two to three drops of aqueous barium nitrate to the mixture.
observations(1 mk) | Inferences(1mk) - Add five drops of dilute nitric (V) acid to the mixture.
observations(1 mk) | Inferences(1mk) - Add to the mixture aqueous sodium hydroxide dropwise until in excess.
observations(1 mk) | Inferences(1mk)
- Add two to three drops of aqueous barium nitrate to the mixture.
- Give the formula of the cation and anion present in substance P.
Cation ......................................... (1/2 mark)
Anion .......................................... (1/2 mark)
- Describe the appearance of substance P. (1 mark)
- You are provided with an organic substance Q. Carry out the following tests and write your observations and inferences in the spaces provided.
- Place about one-third of substance Q on a metallic spatula and ignite it with a Bunsen burner flame.
observations(1 mk) | Inferences(1mk) - Place the remaining amount of substance Q in a boiling tube and add 10 cm3 of distilled water. Heat the mixture and allow it to boil for about 30 seconds. Divide the mixture while still hot into two portions.
- To the first portion, add solid sodium hydrogen carbonate provided.
observations(1 mk) | Inferences(1mk) - To the portion, add two or three drops of acidified potassium manganate (VII).
observations(1 mk) | Inferences(1mk)
- To the first portion, add solid sodium hydrogen carbonate provided.
- Place about one-third of substance Q on a metallic spatula and ignite it with a Bunsen burner flame.
MARKING SCHEME
- Table 1 (PROCEDURE I)
I II III Final burette reading 41.20 19.20 38.00 Initial burette reading 22.00 0.10 19.00 Volume of solution K used (cm3) 19.20 19.10 19.00 - Average = 19.2 + 19.1+ 19.0 = 19.10 cm3 (3 marks)
3 - Moles of Sodium thiosulphate = 19.1 × 0.1
1000
= 0.00191 (1)
Therefore: Moles of Copper ions in 25 cm3 = 0.00191
Moles in 250 cm3 = 0.00191 × 10
= 0.0191 (1)
Concentration of Copper ions =0.0191 × 1000 (1)
25
= 0.764 M (1/2)
- Average = 19.2 + 19.1+ 19.0 = 19.10 cm3 (3 marks)
- Table 2 (PROCEDURE II)
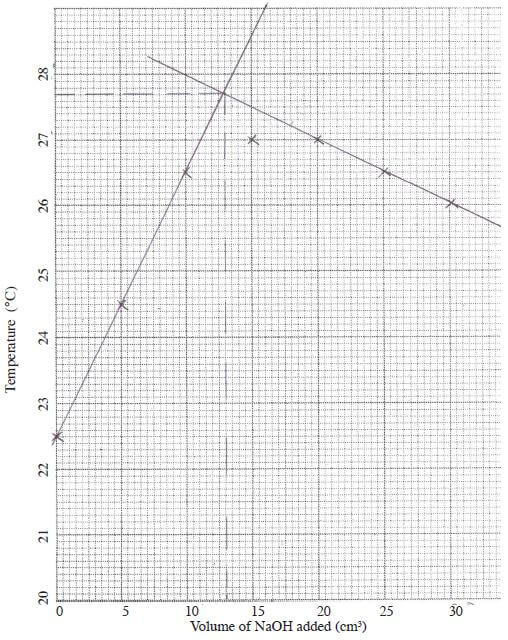
Volume of NaOH added (cm3) 0 5 10 15 20 25 30 Maximum Temperature (°C) 22.5 24.5 26.5 27.0 27.0 26.5 26.0 - Graph
 (3 marks)
(3 marks) -
- 13.0 ± 0.2
1 mark for working
1 mark for value - ▵T = 5.2°C ± 0.1
1 mark
- 13.0 ± 0.2
- ▵H = 33 × 5.2 × 4.2
= 720.72 J (1)
Moles of Cu2+ = 20 × 0.764
1000
= 0.01528 (1/2)
1 mole = 720.721 (1)
0 01528
= -47.2 KJMol-1 (1/2)
- Graph
- QUESTION 2
- White crystalline substance. (1 mark)
- Observations
Colourless liquid condenses on the cool parts of T-Tube leaving behind a white solid(1 mark)
Inferences
Hydrated salt or salt contains water of crystallisation(1 mark) - Observations
Solid dissolves to form colourless solution.(1 mark)
Inferences
P is soluble in water No coloured ions(1 mark) -
- Observations
White PPt formed (1 mark)
Inferences
SO42-, SO32- or CO32- present (2 marks) - Observations
No effervescence or no bubbles (1 mark)
Inferences
SO42-, present or SO32- or CO32- absent (1 mark) - Observations
White PPt (1 mark)
Inferences
Mg2+ present (1 mark)
- Observations
- Cation = Mg2+ or Magnesium ions (1/2 )
anion = SO42- or Sulphate ions (1/2)
- QUESTION 3
- Observations
Burns with a yellow sooty flame or luminous flame. (1 mark)
Inferences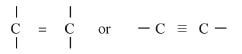
Organic compound with high C:H ration
aromatic compound, long chain organic compound. (1 mark) -
- Observations
Efferescence observed (1 mark)
Inferences
Has a - COOH group or carboxylic/alkanoic acid. (1 mark) - Observations
Decolourised(1 mark)
Inferences
could be an alcohol or has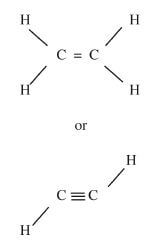 (1 mark)
(1 mark)
- Observations
- Observations
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download KCSE 2014 Chemistry Paper 3 Questions with Marking Scheme.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

