- You are provided with:
- 2.0 g of substance A, labeled solid A
- Solution B, 0.05 M hydrochloric acid.
- Methyl orange indicator.
- Solubility of substance A in water.
- Relative formula mass of substance A.
PROCEDURE I- Place 200 cm3 of tap water in a 250 ml beaker and keep it for use in step (vi).
- Place all of substance A in a dry boiling tube.
- Using a burette, measure 10.0 cm3 of distilled water and add it to the substance A in the boiling tube.
- While stirring the mixture in the boiling tube with a thermometer, boil the mixture using a Bunsen burner, until the temperature rises to 65ºC. Stop warming the mixture.
- Allow it to cool while stirring with a thermometer.
- When the temperature drops to 60ºC, start the stop watch/clock, place the boiling tube in the beaker with hot water prepared in step (i) above.
- Continue stirring and record the temperature of the mixture after two minutes, then thereafter record the temperature of the mixture after every one minute interval and complete table 1. Retain the mixture with the thermometer inside for use in procedure II below.
Table 1
On the grid provided, plot a graph of temperature (vertical-axis) against time. (3 marks)Time(minutes) 0 2 3 4 5 6 7 8 9 10 Temperature (0C) 60 
- Using the graph, determine the temperature (Ts) when 2.0 g of substance A dissolves completely in 10.0 cm3 of distilled water. (1 mark)
- Calculate the solubility of substance A in grams per 100 g water at temperature, Ts. (2 marks)
Using a funnel, transfer all the mixture obtained from Procedure I into a 250 ml volumetric flask. Rinse the boiling tube and the thermometer with about 20 cm3 of distilled water and add the rinses into the volumetric flask. Repeat the rinsing two more times. Add about 100 cm3 of distilled water to the volumetric flask. Shake until all the solid dissolves. Add more distilled water to the mark. Label this as solution A. Fill the burette with solution A. Using a pipette and pipette filler, place 25.0 cm3 of solution B, using solution A. Record your readings in table 2 below. Repeat the titration two more times and complete the table.
Table 2
I II III Final Burette Reading Initial burette Reading Volume of solution A (cm3) used. - Calculate the:
- Average volume of solution A used. (1 mark)
- Number of moles of hydrochloric acid, solution B used. (1 mark)
- Given that two moles of acid reacts with one mole of substance A,
Calculate:- Number of moles of substance A used. (1 mark)
- Concentration of solution A in moles per litre; (1 mark)
- Concentration of solution A in g per litre; (1 mark)
- Relative formula mass of substance A. (1 mark)
- You are provided with solid C. Carry out the following tests and record your observations and inferences in the spaces provided.
Place all solid C in a boiling tube. Add about 15 cm3 of distilled water and shake until all the solid dissolves. Use 2 cm3portions of the solution in the test tube, for each of the tests in (a), (b), (c), (d), (e) and (f).- Add aqueous sodium hydroxide dropwise until in excess.
Observations Inferences (1 mark) (1 mark) - Add aqueous ammonia dropwise until in excess.
Observations Inferences (1 mark) (1 mark) 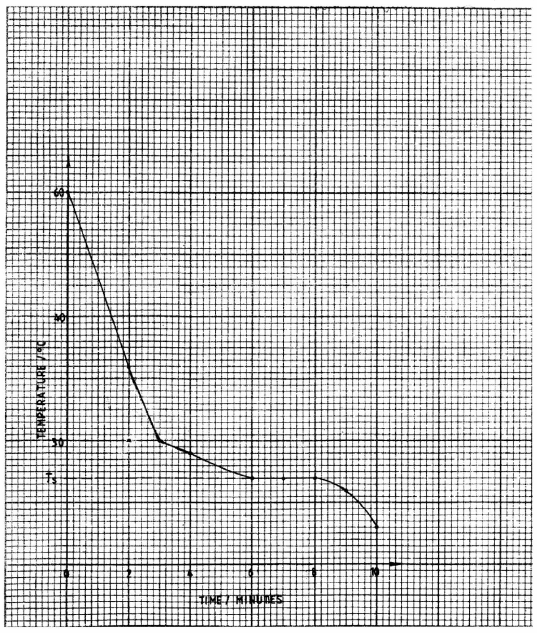
- Add 2 to 3 drops of solution D, aqueous sodium carbonate. (Retain the remaining solution D for use in question 3)
Observations Inferences (1 mark) (1 mark) - Add 2 to 3 drops of dilute hydrochloric acid.
Observations Inferences (1 mark) (1 mark) - Add 2 or 3 drops of aqueous barium chloride.
Observations Inferences (1 mark) (1 mark) - Add 2 or 3 drops of solution E, aqueous lead (II) nitrate.
Observations Inferences (1 mark) (1 mark)
- Add aqueous sodium hydroxide dropwise until in excess.
- You are provided with substance L. Carry out the following tests and record your observations and inferences in the spaces provided. Use about 2 cm3 portions of substance L in a test tube for each of the tests, (a), (b), (c) and (d).
- Add 2 or 3 drops of bromine water.
Observations Inferences (1 mark) (1 mark) - Add about 1 cm3 of acidified potassium dichromate (VI). Warm the mixture.
Observations Inferences (1 mark) (1 mark) - Add about 1 cm3 of solution D, aqueous sodium carbonate provided.
Observations Inferences (1 mark) (1 mark) - Add the piece of magnesium ribbon provided.
Observations Inferences (1 mark) (1 mark)
- Add 2 or 3 drops of bromine water.

MARKING SCHEME
-
(5 marks)Time (minutes) 0 2 3 4 5 6 7 8 9 10 Temperature (°C) 60.0 36.0 30.0 29.0 27.5 27.0 27.0 27.0 26.0 23.0 - Ts is 27(°C). (1) (3 marks)
- Solubility at Ts. (1)
2 g of A in 10 cm3 of H2O
? 100 cm3 of H2O
2 x 100 = 209
10
Table 2
(3 marks)I II III Final burette reading 30.80 38.70 30.70 Initial burette reading 0.00 8.00 0.00 Volume of solution A (cm3) used 30.80 30.70 30.70 -
- Average volume of solution A.
30.7 +30.7 = 30.7 cm3
2 (1 mark) - 25 x 0.05 = 1.25 X 10-3 moles(1 mark)
1000
- Average volume of solution A.
-
- Acid: substance A
2 : 1
= 1.25 x 10-3 = 6.25 x 10-4 (1 mark)
2 - 6.25 x 10-4 moles in 30.7
? moles in 1000
6.25 x 10-4 x 103 =0.02 m
30.7 - Molarity = Conc g/L
RAM
2g - 250
? - 1000
2 x 1000 =8g/L (1 mark)
250 - RFM = conc g/L
molarity
= 8
0.02
= 400 (1 mark)
- Acid: substance A
-
Observations Inferences White precipitate insoluble in excess(1 mark) Probably Ca2+, Mg2+ present
(Accept names of ions) (2 mark)
Observations Inferences No white precipitate No observable change(1 mark) Calcium ions present (Ca2+)(1 mark)
Observations Inferences White precipitate(1 mark) Calcium ions (Ca2+) (1 mark)
Observations Inferences No effervescence(1 mark) CO32- /SO32-,absent(1 mark)
Observations Inferences No white precipitate(1 mark) SO42-(absent) (1 mark)
Observations Inferences White precipitate(1 mark) Cl- present(1 mark)
-
-
Observations Inferences It is not decolourised(1 mark) L must be saturated (1 mark)
Observations Inferences Orange colour persists(1 mark) alcohol absent or R-OH absent(1 mark)
Observations Inferences Effervescence and colourless gas evolved(1 mark) L is acidic or carboxylicacid present
H+, H30+, R-COOH (1 mark)
Observations Inferences Effervescence and colourless gas evolved(1 mark) H+, H30+, R-COOH confirmed.(1 mark)
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download KCSE 2015 Chemistry Paper 3 with Marking Scheme.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

