SECTION A: BIOLOGY (34 marks)
Answer ALL the questions in the spaces provided.
- State the three functions of human blood. (3 marks)
-
- State one example of an organism in the kingdom protoctista,(1 mark)
- Classify maize (Zea mays) into its first two largest taxonomic units. (2 marks)
- Name the organelles observed under a light microscope in plant cells but not in animal cells.(2 marks)
- Explain why a person in a poorly ventilated room with a burning charcoal stove may suffocate to death(3 marks)
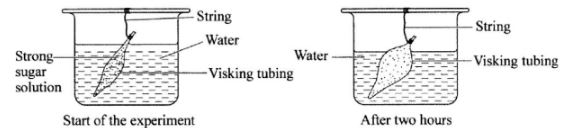
- The diagrams below illustrate a set-up form one students used, to demonstrate a certain physiological process and the result after two hours.
- Name the physiological process that was being demonstrated. (1 mark)
- Explain the observation made after two hours.(3 marks)
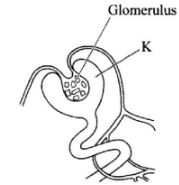
- The diagram below represents part of a human organ.
- Name the structure labelled K(1 mark)
- Explain why contents of K include non-excretory substances in a healthy person.(2 marks)
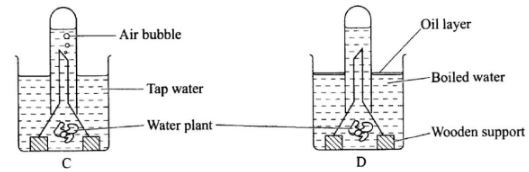
- Form one students set up an experiment to demonstrate a physiological process as shown in the diagrams below.
- Why were bubbles not produced in the set-up labelled D?(2 marks)
- Name the gas collected in the set-up labelled C.(1 mark)
-
- Name the branch of biology that deals with the study of animals.(1 mark)
- Give two reasons for classifying living organisms (2 marks)
-
- Differentiate between excretion and egestion.(2 marks)
- How does the liver help to maintain a constant body temperature in human beings?(2 marks)
- State two causes of kidney stones.(2 marks)
-
- Apart from thermoregulation, state two other roles of the skin in homeostasis. (2 marks)
- How does amoeba maintain osmotic pressure when placed in a hypotonic solution?(2 marks)
SECTION B: CHEMISTRY (33 marks)
Answer ALL the questions in the spaces provided
- The diagram below shows some changes in the physical states of matter. Study it and answer the questions that follow.
- Name the changes represented by letters R and S.(2 marks)
- Name the method used to separate coloured substances in a dye.(1 mark)
- Magnesium burns in air with a bright flame.
- State another observation made when magnesium burns in air.(1 mark)
- Write an equation for the reaction.(1 mark)
-
- Write a word equation for the reaction between dilute hydrochloric acid and calcium hydrogen carbonate.(1 mark)
- Name the acid which is commonly used in car batteries.(1 mark)
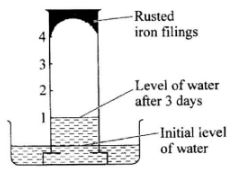
- The diagram below shows the results obtained when wet iron filings in a gas jar inverted over water were left standing for 3 days.
Given that the wet iron filings were in excess, what would be the effect of leaving the set up to stand for a further 3 days? (1 mark) - The diagram below shows a reduction - oxidation process. Study it and answer the questions that follow
- Write an equation for the reaction between dry hydrogen gas and hot copper (II)oxide.(1 mark)
- In the process above, which substance undergoes oxidation? Explain.(2 marks)
- Name the substance that burns at X?(1 mark)
- The table below gives information about substances N, P, Q and R.
Substances
Melting point
(ºC)Boiling Point
(ºC)Electrical conductivity when Solid State Molten Dissolved in
WaterN -115 -85 Poor Poor Good P 801 1467 Poor Good Good Q 98 890 Good Good Good R -117 78.5 Poor Poor Poor - Select a substance that is likely to be hydrogen chloride.(1 mark
- Which letter represents a substance that is likely to have:
- metallic bonding (1 mark)
- ionic bonding(1 mark)
- State how the following substances conduct electricity.
- Molten calcium chloride. (1 mark)
- Graphite.(1 mark)
-
- State the purpose of the pH scale. (1 mark)
- Hydrochloric acid is a strong acid. Explain the meaning of a strong acid.(1 mark)
- Dilute hydrochloric acid was reacted with solid calcium carbonate in a test tube. Write a balanced chemical equation for the reaction. (1 mark)
- Give two disadvantages of washing clothes in hard water using soapy detergents. (2 marks)
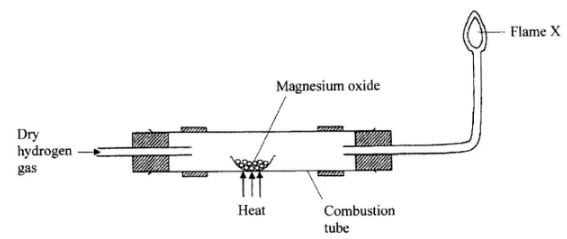
- The diagram below illustrates an experiment where dry hydrogen gas is passed over heated magnesium oxide.
- State the observation that is made in the combustion tube. (1 mark)
- Explain the observation made in (a) above, (1 mark)
- What substance burns at flame X? (1 mark)
-
- Name the type of reaction that occurs when a solution of lead (II) nitrate is added to a solution of sodium sulphate (in a boiling tube). (1 mark)
- Write a balanced equation for the reaction that occurs when crystals of sodium nitrate are heated in a test tube. (1 mark)
- Give the meaning of an acid salt. (1 mark)
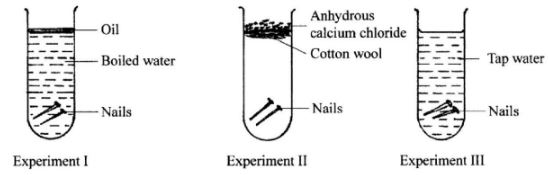
- Three experiments were set up as shown below to investigate the conditions necessary for rusting to occur.
- After three days, only the nails in experiment III had rusted. Why didn't rusting occur in experiment I and II?
- What would be the effect of using salty water instead of tap water in experiment III? (1 mark)
- Complete the table below by stating the type of oxides formed when the following substances are burnt in air. (2 marks)
Substance Typ of oxide Hydrogen Neutral Phosphorus Magnesium
- After three days, only the nails in experiment III had rusted. Why didn't rusting occur in experiment I and II?
SECTION C: PHYSICS (33 marks)
Answer ALL the questions in the spaces provided
- When thirty (30) drops of a liquid were released from a burette, the liquid level changed from the 25 ml mark to the 40 ml mark. Determine the volume of each drop. (2 marks)
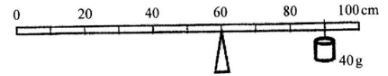
- The figure below shows a uniform metre rule pivoted at the 60 cm mark. The rule is balanced when a 40g mass is supported at the 90cm mark.
- Show on the diagram the position of the centre of gravity of the metre rule. (1 mark)
- Determine the mass of the metre rule. (2 marks)
- A student in a room observed a beam of sunlight entering into the room from a hole on the roof. The student noted that dust particles illuminated by the beam were moving in random motion. Explain how this motion was caused. (2 marks)
-
- Define the term temperature.(1 mark)
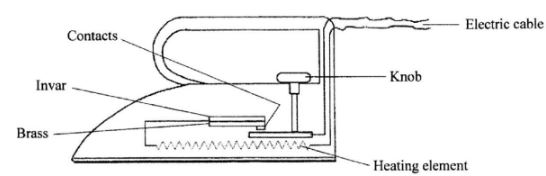
- The figure below shows an electric iron box in which a brass-invar bimetallic strip is used to control the temperature.
Given that brass expands more than invar, describe how the bimetallic strip controls the temperature of the iron box. (2 mark
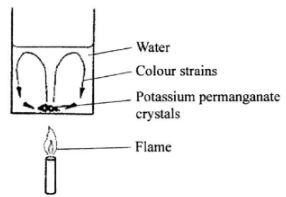
- The figure below shows a crystal of potassium permanganate at the bottom of a beaker The figure below containing some water.
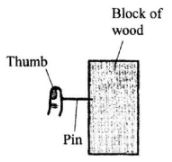
It is observed that when the beaker is heated from the bottom, strains of colour rise up from the crystal and curve out as shown. Explain the observation.(3 marks) - The figure below shows a pin being pushed into a block of wood using a thumb.
Explain why the pin penetrates the wood and not the thumb. (2 marks) - The length of a spring is 20 cm. When it is used to support a mass of 0.4 kg its new length is 20.8cm. Determine the spring constant (take acceleration due to gravity, g - 10 ms).(3 marks)
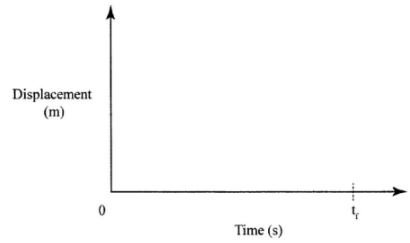
- A stone is thrown vertically upwards. On the axes provided sketch the displacement-time graph for the motion of the stone from the time it is thrown to the time, t, when it reaches the maximum height. (2 marks)
-
- A block of wood is pulled along a horizontal surface. State one factor that determines, the magnitude of the frictional force between the block and the surface. (1 mark)
- The figure below shows a vertical glass tube containing a liquid. (1 mark)
- State the reason for the meniscus in terms of molecular forces. (2 marks)
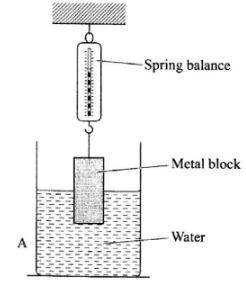
- The figure below shows a metal block suspended from a spring balance and partially immersed in water.
- State what will be observed in the reading of the balance if the block is lowered further into the water. (1 mark)
- Explain your answer in (a). (2 marks)
- When a drop of water is placed on a clean metal surface it wets the surface. Explain this observation in terms of the forces involved. (3 marks)
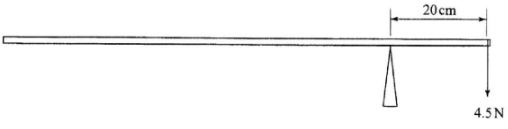
- The figure below shows a uniform metre rule pivoted at the 20cm mark and balanced by a weight of 4.5N.
Determine the weight of the metre rule. (3 marks)
Download KCSE 2016 General Science Paper 1 Questions with Marking Scheme.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students