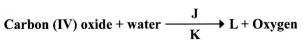SECTION A: BIOLOGY (34 marks)
Answer all the questions in this Section in the spaces provided.
- State the three functions of blood plasma. (3 marks)
-
- Name one organism in the Kingdom Monera. (1 mark)
- Classify the domestic dog (Canis familiaris) into the following taxonomic units:
- phylum (1 mark)
- class (1 mark)
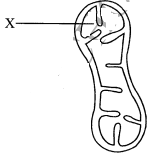
- The diagram below shows a cell organelle found in an animal cell.
- Name the organelle illustrated above (1 mark)
- Identify the part labelled X (1 mark)
-
- State the function of the organelle illustrated. (1 mark)
- How is the organelle illustrated above structurally adapted to its function? (2 marks)
- State three characteristics of respiratory surfaces in animals. (3 marks)
- State three factors that increase the rate of diffusion. (3 marks)
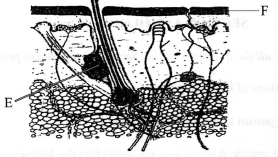
- The diagram below shows a section of the human skin.
-
- Name the part labelled E (1 mark)
- Give one function of the structure labelled E (1 mark)
- How is the part labelled F adapted to its function? (2 marks)
-
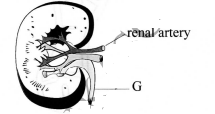
- The diagram below represents a section of a human kidney.
-
- Name the structure labelled G. (1 mark)
- Give the function of the structure labelled G (1 mark)
- State two ways of treating kidney failure. (2 marks)
-
- Give three structural factors that increase the rate of transpiration in plants. (3 marks)
- State one difference between movement and locomotion. (1 mark)
- Below is a word equation of a process in plant nutrition.
-
- Name the product labelled L (1 mark)
- State the requirements labelled J and K (2 marks)
- Explain the importance of the product L. (2 marks)
-
SECTION B: CHEMISTRY (33 marks)
Answer all the questions in this Section in the spaces provided
- Dilute Sulphuric(VI) acid was added to A, which is a compound of magnesium. A reacted with the acid to form a colourless solution B and a colourless gas C which formed a white precipitate with calcium hydroxide solution.
- Identify:
- Compound A (1 mark)
- Solution B (½ mark)
- Gas C (½ mark)
- Write an equation for the reaction that took place between compound A and the acid. (1 mark)
- Identify:
-
- Describe how a sample of oil can be extracted from macadamia seeds in a Chemistry laboratory. (2 marks)
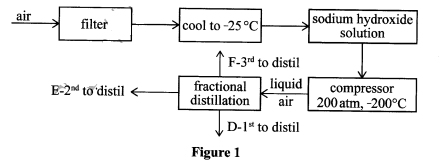
- Figure 1 shows the steps followed during fractional distillation of liquid air.
- Name the process that takes place at the filter. (1 mark)
- State the role of sodium hydroxide solution in the preparation of liquid air. (1 mark)
- Identify substance D. (½ mark)
- State one use of substance E. (½ mark)
- Draw and label the structure of G. (2 marks)
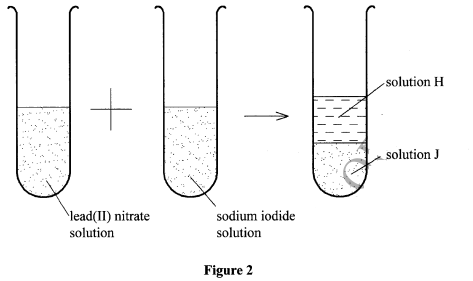
- Figure 2 is an illustration of one of the methods used to prepare salts.
- Name solid J. (1 mark)
- Name the method of salt preparation demonstrated in Figure 2. (1 mark)
- In terms of structure and bonding explain the following statements.
- Copper is used to make electrical cables. (1 mark)
- Solid sodium chloride does not conduct heat and electricity. (2 marks)
- Use the information in Table 1 to answer the questions that follow.
Table 1
K L M N KO no reaction no reaction no reaction no reaction NO KO+N LO+N MO+N no reaction LO KO+L no reaction MO+L no reaction MO KO+M no reaction no reaction no reaction - Arrange the elements in order to reactivity starting with the most reactive. (2 marks)
- What property of the elements is displayed in Table 1? (1 mark)
- Explain the following statements:
- Group VIII elements are said to be inert. (1 mark)
- Magnesium and silicon are solids at room temperature, yet silicon has a higher melting point than magnesium.
(3 marks)
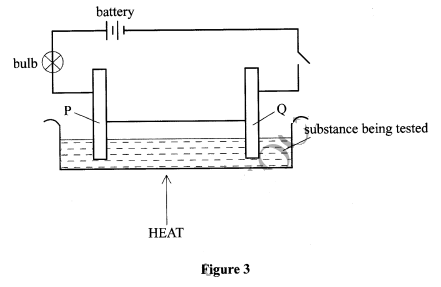
- Figure 3 shows a set up that is used to investigate the effect of an electric current on various substances.
- Identify the electrodes labelled
- P (½ mark)
- Q (½ mark)
- What observations will be made if lead(II) bromide was the substance under investigation. (1 mark)
- Identify the electrodes labelled
- Explain how water hardness can be removed using the ion-exchange method. (2 marks)
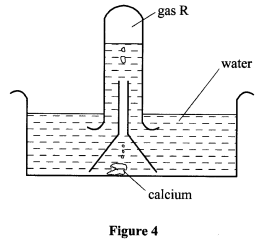
- The set up in Figure 4 was used to investigate the reaction of calcium with cold water.
- Name gas R. (1 mark)
- Explain the observation that was made when a few drops of phenolphthalein indicator were added to the resulting solution. (1 mark)
- If magnesium ribbon was used in place of calcium, explain why it was necessary to clean the magnesium with steel wool before its reaction with water. (1½ mark)
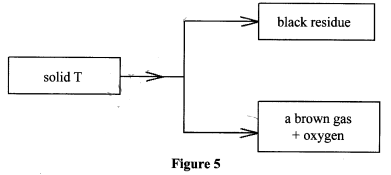
- Study Figure 5 and answer the questions that follow.
- Name solid T. (1 mark)
- Identify the brown gas.(1 mark)
SECTION C: PHYSICS (33 marks)
Answer all the questions in this Section in the spaces provided.
- State one reason why students should not carry or eat food in the laboratory (1 mark)
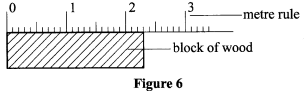
- Figure 6 shows a metre rule being used to measure the length of a block of wood.
Record the length of the block of wood. (1 mark) - Complete the Table 2 below.
Table 2
Physical Quantity SI Unit Symbol of Unit Mass Newton - Define the term Power. (1 mark)
- Two students A and B are taking milk using a straw. A is standing on top of a high mountain while B stands at the foot of the mountain. State with a reason which student takes the milk more easily. (2 marks)
- Explain why a gas occupies the whole space of the container in which it is placed. (2 marks)
-
- A student observed that gaps between rails along a railway line were larger in the morning than in the afternoon. Explain the observation. (2 marks)
- State the purpose of the constriction in a clinical thermometer (1 mark)
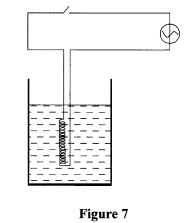
- Figure 7 shows an immersion heater being used to heat water in a jug and a thermometer placed in the water with its bulb below the heating coil.
It was observed that when the water started to boil the thermometer reading was lower than the boiling point of water. Explain this observation (2 marks) - A uniform plank of wood of length 5 m is pivoted at its centre. A girl of mass 32 kg sits at one end of the plank. Determine how far from the pivot a boy of mass 40 kg should sit in order to balance the plank. (3 marks)
-
- A ball rests on a level floor. Name its state of equilibrium. (1 mark)
- State how the area of the supporting base affects the stability of the object.
-
- Define "elastic limit" (1 mark)
- A spring supporting a weight of 30N extends by 1.5 cm. Determine the spring constant. (3 marks)
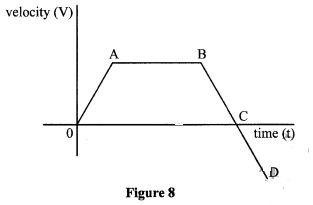
- Figure 8 shows the velocity time graph for a vehicle.
Describe the motion of the vehicle in the regions. (3 marks)
AB .....
BC .....
CD..... -
- Define inertia. (1 mark)
- Explain how striking a matchstick on a matchbox causes fire. (2 marks)
-
- State the law of flotation. (1 mark)
- Explain why an object weighs more in air than when fully immersed in water. (3 marks)

MARKING SCHEME
SECTION A: BIOLOGY (34 marks)
-
- Acts as a transport medium for food substances/waste products (from tissues to excretory organs);
- Transport of hormones (to target organs):
- Transfer of heat within the body
- ; Provides medium in which (soluble) substances, ions are transported;
3x1=(3 marks)
-
-
- Bacterium;
- Blue algae;
1x1=(1 mark)
-
- Phylum Chordata; (1 mark)
- Class - Mammalia; (1 mark)
-
-
- Mitochondrion; (1 mark)
- Matrix; (1 mark)
-
- Acts as a site for energy synthesis/respiration; (1 mark)
- Inner membrane is folded into infoldings/cristae); to increase surface area for attachment of respiratory enzymes (increasing surface area for respiration); (2 marks)
-
- Moist; to dissolve respiratory gases for faster gaseous exchange;
- Highly vascularized/supplied with dense network of blood capillaries for efficient transportation of respiratory gases;
- Lined with one-cell-thick/thin epithelia to reduce the diffusion distance;
3x1=(3 marks)
-
- Increased/high temperature (to a given optimum);
- Increasing surface area to volume ratio;
- Increasing concentration gradient;
- Reducing the sizes of diffusing particles/using smaller diffusing particles;
3 x 1 = (3 marks)
-
-
- Sebaceous gland; (1 mark)
- Produces sebum which is antiseptic and prevents cracking/drying of the skin/keeps it moist/supple;
(1 mark)
-
- Is made up of dead cells to protect inner (delicate) parts from mechanical damage, microbial attacks, desiccation, etc;
- Is perforated to allow for elimination of (nitrogenous) wastes;
- Lined with hair for insulation/thermoregulation;
2x 1= (2 marks)
-
-
-
- Ureter; (1 mark)
- Drains urine into the urinary bladder; (1 mark)
-
- Dialysis;
- Kidney transplant;
2x1= (2 marks)
-
-
- Broad leaf surface;
- Thin cuticle;
- Lack of/absence of epidermal hair;
- Increased number/numerous stomata on the upper leaf surface;
3x1 = (3 marks)
- Locomotion involves the displacement/movement of the entire body (of organism) from one place/point to another while movement may only be limited to (some) parts of an organism (for instance, roots/shoot) or the entire organism; (1 mark)
-
-
- Glucose; (1 mark)
- Light energy;
Chlorophyll; (2 marks)
(Either for J and K)
- It is oxidized/broken down; to release energy during respiration; (2 marks)
-
SECTION B: CHEMISTRY(33 marks)
-
-
- Magnesium carbonate (MgCO3) (1 mark)
- Magnesium sulphate solution (MgSO4(aq)) (½ mark)
- Carbon(IV) oxide / Carbon(IV) oxide (CO2) (½ mark)
- MgCO3(s)+H2SO4(aq) → MgSO4(aq) + CO2(g) +H2O(l) (1 mark)
-
-
-
- Crush the seeds in a mortar using a pestle; (½ mark)
- Continue crushing the seeds while adding acetone! propanone a little at a time; (½ mark)
- Decant the resulting solution into an evaporating dish/basin; (½ mark)
- Leave the solution in the sun for some time. Propanone evaporates because of its low boiling point. The residue liquid is the oil. (½ mark)
-
- Electrostatic precipitation (1 mark)
- NaOH(aq) is an alkali hence it absorbs Carbon(IV) oxide from the air. (1 mark)
- D-Nitrogen. (½ mark)
- E
- used in filling electric light bulbs;
- used as an insulator during welding of metals. (½ mark)
- (Any 1 correct @ 1mk)
-
-
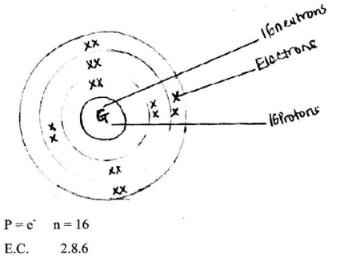
- Mass number of G is 32 (1 mark)
Atomic number of G is 16 (1 mark) - Electronic configuration of 2.8.6
(2 marks)
- Mass number of G is 32 (1 mark)
-
- J-Lead(II) iodide
- Precipitation / double decomposition
-
- Copper is used in electrical appliances due to its good electrical conductivity because of presence of delocalized electrons. (1 mark)
- Sodium chloride is an ionic compound in which the ions are immobile when in the solid state hence it does not conduct heat and electricity (2 marks)
-
- K, M, L,N
→Decreasing reactivity (2 marks) - Competition for combined oxygen/ reduction (1 mark)
- K, M, L,N
-
- Group VIII elements are inert since the highest occupied energy level is completely filled with electrons thus electronically stable. (1 mark)
- Magnesium has a giant metallic structure in which the positive ✓½ nucleus are immersed in a sea/cloud of electrons while silicon ✓½ has a giant atomic structure in which the atoms are joined ✓1 by strong covalent bonds hence silicon is harder than Magnesium ✓½ metal hence the high melting point. ✓½
-
-
- P-Anode (½ mark)
- Q - Cathode (½ mark)
-
- Brown fumes at the electrode P/ anode; (½ mark)
- grey pellets at electrode Q/cathode; (½ mark)
-
- Ion-exchange process:
- Hard water is passed through a column filled with a complex sodium compound (sodium permuttit) /ion exchanger; (1 mark)
- The Ca2+ and Mg2+ ions in the hard water are precipitated and remains in the column while the sodium ions from the column comes out with the water hence becoming soft. (1 mark)
-
- Gas R-Hydrogen (H2) (½ mark)
- When a few drops of Phenolphthalein indicator are added to the resulting solution, the solution changed/turned pink. (½ mark) This is because Calcium reacted with water to form Calcium hydroxide which is alkaline hence the pink colour. (½ mark)
- Magnesium reacts with air to form a layer of Magnesium oxide which has to be removed before it can react with water.
-
- Copper(II) nitrate / Cu(NO3)2 (1 mark)
- Nitrogen(IV) oxide /NO2 (1 mark)
SECTION C: PHYSICS (33 marks)
- To avoid eating contaminated food. ✓1 (1 mark)
- 2.3 cm ✓1 (1 mark)
-
(2 marks)Physical Quantity SI Unit Symbol of Unit Mass Kilogram ✓½ Kg ✓½ Weight ✓½ Newton N ✓½ - The rate of doing work. ✓1 (1 mark)
- B ✓1
Atmospheric pressure is higher at the foot of the mountain hence the milk gets into the straw much more easily. ✓1
(2 marks) - The intermolecular forces very weak.✓1hence molecules move randomly in all directions. (2 marks)
-
- In the afternoon it is hotter than in the morning.✓1 hence rails expand more reducing the gaps. ✓1 (2 marks)
- To prevent the liquid from flowing back to the bulb. ✓1 (1 mark)
- Water above the coil was heated by convection currents; ✓1 while below the coil water is heated by conduction but water is a poor conductor of heat. ✓1 (2 marks)
- Sum of clockwise moments = sum of anticlockwise moments. ✓1
40x = 32 x 2.5 ✓1
x = 32 x 2.5
40
= 2.0m ✓1 (3 marks) -
- Neutral ✓1 (1 mark)
- The wider the supporting base the more stable the object is ✓1 (1 mark)
-
- Force beyond which the extension is not proportional to the applied force.✓1 (1 mark)
- F = Ke✓1
1.5k = 30 ✓1
k=20 Ncm−1 or 2000 Nm−1 ✓1 (3 marks)
- AB - vehicle moves with uniform velocity. ✓1
BC - vehicle decelerates uniformly until it comes to rest ✓1
CD-vehicle moves in the opposite direction with increasing velocity ✓1 (3 marks) -
- Resistance to change the state of motion of a body. ✓1 (1 mark)
- By striking there is friction between the matchstick and the matchbox✓1friction causes/generates heat. ✓1
(2 marks)
-
- A floating body displaces its own weight of the fluid in which it floats. ✓1 (1 mark)
- When in air the upward force (upthrust) is negligible ✓1 when in water the object experiences an upthrust ✓1 hence when in water the net downward force is less than when ✓1 in air (when only the weight acts on the body) (3 marks)
Download KCSE 2017 General Science Paper 1 with Marking scheme.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students