INSTRUCTIONS TO CANDIDATES
- Write your name and Index Number in the spaces provided above.
- Sign and write date of examination in the spaces provided above.
- Answer ALL questions in the spaces provided.
- Mathematical tables and electronic calculators may be used.
- All working MUST be clearly shown where necessary.
-
- Define the term half-life as used in radioactivity. (1mk)
- 100g of radioactive substance was reduced to 12.5g in 15.6years. Calculate the half-life of the substance. (2mks)
- You are provided with water and usual laboratory apparatus. Describe how you would fully separate solid lead (II) carbonate from a mixture of iron fillings, lead (II) carbonate and sodium carbonate. (3mks)
- In order to determine the molar heat of neutralization of sodium hydroxide, 100cm3 of 1M NaOH and 100cm3 of 1M HCI both at the same initial temperature were mixed and stirred continuously with a thermometer. The temperature of the resulting solution was recorded after every 30 seconds until the highest temperature was attained. Thereafter the temperature of the solution was recorded for further two minutes.
- Write the ionic equation for the reaction which took place. (1mk)
- The sketch below was obtained when the temperature of the mixture were plotted against time. Study it and answer the questions that follow.
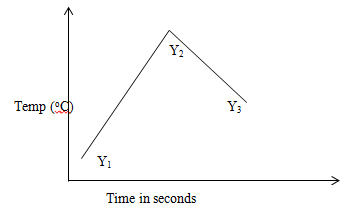
- What is the significance of point Y2 (1mk)
- Explain the temperature change;
Between Y1 and Y2 (1mk)
Between Y2 and Y3 (1mk)
- Dry chlorine gas was passed through two pipes of coloured cotton cloth as shown below.
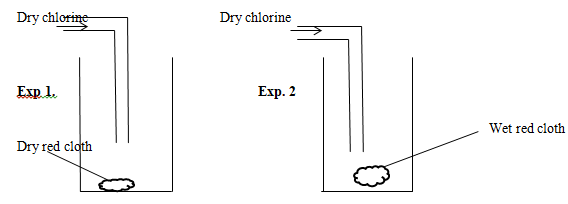
- State what is observed in each of the experiment;
Experiment 1 (1mk)
Experiment 2 (1mk) - Explain your observation using an equation. (1mk)
- State what is observed in each of the experiment;
- Two elements A and B have electronic configuration 2.8.3 and 2.6 respectively.
- To which group and period does element B belong? (1mk)
- If the two react, what is the formula of the compound they form? (1mk)
- Iron fillings react with steam according to the equation given below.
3Fe(s) + 4H2O(g) ⇌ Fe3O4(s) + 4H2(g)
State and explain the effects of each of the following on the equilibrium.- Increase in pressure (2mks)
- Addition of magnesium ribbon to the equilibrium mixture. (2mks)
- Unknown substances had PH values as shown in the table below.
State which substance is likely to be;Substance PH values A 6.0 B 2.0 C 8.0 - Lemon juice (1mk)
- Phosphoric (v) acid (1mk)
- Identify a substance that would be a better electrolyte? (1mk)
- In an experiment to study diffusion of gases, the following set up was used.
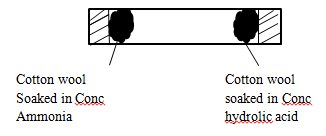
- State and explain the observations made in the experiment. (2mks)
- Write an equation for the reaction that occurs in the experiment. (1mk)
- An electric current was passed through molten potassium fluoride using inert electrodes.
- Name the products at;
Anode (1mk)
Cathode (1mk) - Write an equation for the reaction at the anode. (1mk)
- Name the products at;
- During the extraction of copper and zinc from their ores, some of the processes include;
- Crushing
- Mixing of the crushed ore with oil and water and bubbling air through it.
-
- Name the process (ii) above. (1mk)
- What is the purpose of process (ii) above? (1mk)
- Bronze is an alloy of copper and another metal. Identify the other metal. (1mk)
-
- Name another gas which is used together with oxygen in welding. (1mk)
- The structure of ammonium ion is shown below.
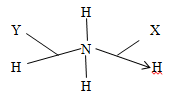
- Name the type of bond represented by X and Y
X………………………… (1mk)
Y………………………….. (1mk) - How many electrons are used in bonding in the ammonium ion? (1mk)
- Name the type of bond represented by X and Y
- A dibasic acid H2C2O4nH2O of concentration 6.3g/dm3 was titrated against NaOH solution. 25cm3 of the acid solution required 15.6cm3 of 0.16MNaOH for complete neutralization. Calculate the value of n in the formula. (H=1, O=16, C=12) (3mks)
- The table below shows the solubility of potassium nitrate and potassium chlorite at various temperatures.
A mixture of salts contains 20g of KNO3 and 18g of KCIO3 in 100g of water at 50ºC.Salt Solubility at various temperatures 50ºc 20ºC KNO3 86g 31g KCIO3 18g 8g - State the method which may be used to separate the mixture. (1mk)
- If the mixture was cooled from 50ºC to 20ºC, state and explain what would be observe. (2mk)
-
- Name the following organic compounds.
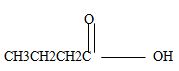
CH3CH2CH2CH3 (1mk) - Below is a simple representation of a soap molecule.
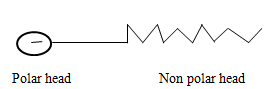
Using the structure above show how soap removes an oil smear from the fabric below.(2mks)
- Name the following organic compounds.
- Explain how a sample of lead(ii) chloride can be prepared using the following reagents.
- Dilute nitric (v) acid
- Dilute hydrochloric acid
- Lead (ii) carbonate (3mks)
- The diagram below represents a set up used to react magnesium with steam. Study it and answer the questions that follow below.
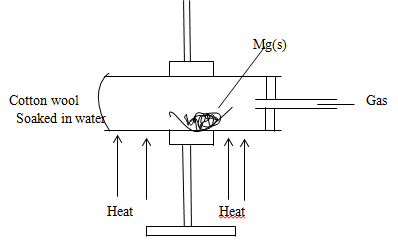
- State the observation made in the combustion tube. (1mk)
- Why would it not be advisable to use potassium in place of magnesium In the above set up. (1mk)
- Explain why cotton wool is heated prior to heating magnesium (1mk)
- The scheme below shows some reaction sequence starting with solid M.
H2SO4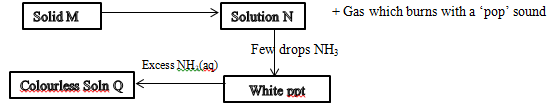
- Name solid M (1mk)
- Write the formula of a complex ion present in solution Q (1mk)
- Write an ionic equation of the reaction between Barium nitrate and solution N.(1mk)
-
- Below are standard reduction potentials of 3 electrodes.
Fe2+(aq) + 2e Fe(s) -0.44v
Zn2+(aq) +2e Zn(s) -0.76v
Sn2+(aq) + 2e Sn(s) -0.14v
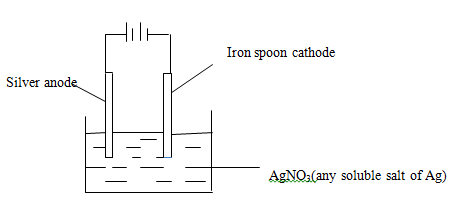
Calculate the electromotive force of a cell formed between Fe/Fe2+ half-cell and Zn/Zn2+ half-cell. (2mks) - Draw a clearly labeled diagram of a set up you would use to electroplate an iron spoon with silver metal. (2mks)
- Below are standard reduction potentials of 3 electrodes.
-
- Name the process of extracting Sulphur. (1mk)
- What is the role of super-heated water? (1mk)
- State two uses of sulphur (1mk)
- The diagram below shows how carbon (ii) oxide can be prepared starting with carbon (iv) oxide and solid W. study it and answer the questions that follow.
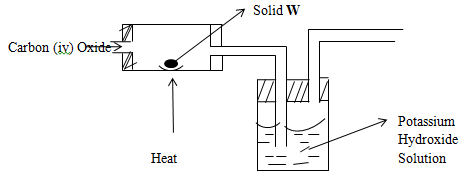
- With reasons, state a suitable location where such an experiment should be rightly conducted. (1mk)
- What is the purpose of concentrated potassium hydroxide? (1mk)
- Identify solid W (1mk)
-
- Explain how you would separate a mixture of nitrogen and oxygen. (2mks)
- Draw a well labeled diagram to show the percentage composition of oxygen in air can be determined. (2mks)
- Use the information below to answer the questions that follow.
H2(g) +¹₂O2 → H2O(l) ∆H1= -286KJ/Mol
C(s) + O2(g) → CO2(g) ∆H2 = -384KJ/Mol
C(s) +4H2(g) +¹₂O2(g) → C3H7OH ∆H3 = -2686.6KJ/Mol- Define ‘enthalpy of formation’ (1mk)
- Determine the molar enthalpy of formation of propanol. (2mks)
- Most natural water occurs as permanent hard water or temporary hard water.
- Name two compounds that cause;
- Temporary hardness (1mk)
- Permanent hardness (1mk)
- How is temporary hardness removed from water? (1mk)
- State one disadvantage of using hard water in boilers. (1mk)
- Name two compounds that cause;
- Both Sodium and Aluminum are metals in period 3 yet sodium has much lower melting point than aluminum. Explain. (2mks)
- Determine the values of X and Y in the equation below.
23692U + xYBa → 9236Kr + 10Z + Energy
x………………………………………. Y……………………………….. (1mk) - State two effects of emitting SO2 in the environment. (1mk)

MARKING SCHEME
-
- Time taken for half the amount of radio isotope to decay (1mk)
- 1 2 3
100 → 50 → 25 → 12.5 (1mk)
3=15.6years
1=15.6 =5.2 years (1mk)
3
-
- Pass a magnet through the mixture to attract iron which is magnetic. Lead (ii)carbonate and sodium carbonate remains. (½mk)
- Add water to the mixture of sodium carbonate and lead(ii)carbonate and stir. (1mk)
- Sodium carbonate dissolves unlike lead(ii)carbonate
- Filter to obtain lead(ii)carbonate as the residue to rinse it, allow to dry (½mk)
- Run water through the residue to rinse it, allow to dry. (½mk)
(reject : dissolve the mixture in water)
-
- H+(aq) +OH-(aq) → H2O(l) (1mk)
- Y2 complete neutralization/end point (1mk)
Y1 and Y2 neutralization is taking place producing heat (1mk)
Y2 and Y3 reaction has come to end; products are cooling releasing heat to the surrounding (1mk)
-
- Exp 1-No change on the dry cloth (½mk) due to the absence of hypochlorous acid (½mk)
Exp 2-The cloth turns to white /bleached due to the presence of chloric (i)acid/hypochlorous acid. (1mk) - CI2(g) +H2O(l) → HCI(aq) +HOCI(aq) (1mk)
Dye +HOCI(aq) → (Dye +O) +HCI(aq) (1mk)
- Exp 1-No change on the dry cloth (½mk) due to the absence of hypochlorous acid (½mk)
-
- Group VI, period 2 (1mk)
- A2B3 (1mk)
-
- No effect on position of equilibrium. The volume of gaseous reactants and products is the same. (1mk)
- There is more formation of iron(iii)oxide, magnesium is more reactive than iron thus reacts with steam at the expanse of iron and this lowers concentration of water molecules. (1mk)
- Equilibrium shifts to the left, steam will react with Mg i.e remove steam. (1mk)
-
- A (1mk)
- B (1mk)
- C (1mk)
-
- A white solid was formed (1mk) inside combustion tube closer to the cotton wool soaked in concentrated hydrochloric acid. Ammonia is less dense hence diffuse faster. (1mk)
- NH3(g) +HCI(g) → NH4CI(s) (1mk)
-
- Fluorine (1mk)
Potassium (1mk) - Anode 2F-(l) → F2(g) +2e- (1mk)
Cathode 2K+(I) +2e- → 2K(I)
- Fluorine (1mk)
- Froth floatation
Concentrating the mineral ore by making impurities to sink at the bottom. (1mk)
Tin (1mk) - Acetylene /Hydrogen (1mk)
-
- Dative bond/Coordinate bond (1mk)
Covalent bond (1mk) - 8 electrons (1mk)
- Dative bond/Coordinate bond (1mk)
- Value of n in the formula
Moles of NaOH=15.6*0.16 =0.002496moles (½mk)
1000
Mole ratio of acid: base =1:2
Thus moles of acid =0.002496 =0.001248moles (½mk)
2
If 25cm3 → 0.002428moles
1000cm3 → 1000*0.001248 =0.04992M (½mk)
25
Thus RFM; 6.3 = 126 (½mk)
0.04992
H2C2O4.nH2O=126,
90+18n=126,
18n=36
n=2 (1mk) -
- Fractional crystallization (1mk)
- Observation 10g of potassium Chlorate (18-8=10g)crystallizes while no KNO3 crystallizes. (1mk)
Reason : The solubility of one salt has no effect on the solubility of other salt. (1mk)
-
- Butanoic acid (1mk)
Butane (1mk) -
- Butanoic acid (1mk)
- Add excess lead(ii) carbonate to the nitric(v) acid: warm to form lead(ii) nitrate, water and carbon (iv)oxide (1mk)
PbCO3(s) +2HNO3(aq) → Pb(NO3)2(aq) + H2O(I) +CO2(g)- Filter off excess lead(ii) carbonate (½mk)
- To the filtrate, add dilute hydrochloric acid and stir (½mk)
- Filter to obtain lead(ii)chloride as residue. All the residue to dry (½mk)
-
- Magnesium burns with a white bright flame. White solid formed
White residue is formed (1mk) (Any one) - Potassium will react explosively (1mk)
- If magnesium is heated first it reacts with air in the setup, to generate steam that reacts with magnesium. (1mk)
- Magnesium burns with a white bright flame. White solid formed
-
- Zinc/Zinc metal (1mk)
- Zn(NH3)2+4 (1mk)
- Ba2+(aq) +SO42-(aq) → BaSO4(s) (1mk)
-
- E.M.F=Ereduction-Eoxidation
(-0.44)-(-0.76)
-0.44 + 0.76=+0.32V (2mks) -
- E.M.F=Ereduction-Eoxidation
-
- Frasch process (1mk)
- To melt sulphur deposit (1mk)
-
- Vulcanization
- Manufacture of sulphuric acid
- Any other/Sulphur based drugs (Any two correct) (1mk)
- Fume chamber (1mk)
To dissolve /remove carbon (iv) oxide (1mk)
Carbon /coke (1mk) -
- Compress to 2000 ATM then cool to -200ºC. Then carry out fractional distillation.(2mks)
- Any appropriate diagram but labeled (2mks)
-
- Heat change that occurs when 1M of a substance is made from its constituent elements under STP conditions. (1mk)
- Heat of combustion = -2296 + -2686 (1mk)
= -4976KJmol-1 (1mk)
-
-
- Magnessium hydrogen carbonate and calcium hydrogen carbonate (1mk)
- Calcium sulphate, calcium chloride, magnesium sulphate, magnesium chloride. (1mk)
- By boiling during which magnesium or calcium ions are precipitated out as their respective carbonate (1mk)
- Results to the formation of kettle fur/furring which reduces heat and electrical conductivity of the boilers hence reduced efficiency. (1mk)
- Formation of scum with soap which leads to soap wastage and destruction of some fabrics such a as silk (1mk)
-
- Aluminium has more protons in nucleus than sodium; leading into higher nuclear charge hence nuclear attraction; thus leads to stronger metallic bond in aluminium than in sodium.
- Value of X=143 (½mk)
Y=56 (½mk) -
- Cause respiratory problems (½mk)
- Leads to formation of acidic rain which has adverse effects on living organism.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions and Answers - Samia Joint Mock Examination 2021/2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

