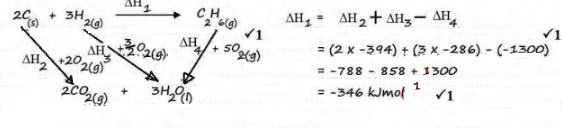CHEMISTRY
Paper 1
Instructions to candidates
- All working MUST be clearly shown.
- KNEC mathematical tables and silent non programmable electronic calculators may be used.

Questions
-
- Give two reasons why luminous flame is not used for heating purposes in the laboratory. (2marks)
- Explain how the hotness of a Bunsen burner flame can be increased. (1mark)
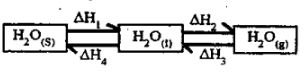
- The scheme below shows the energy changes that are involved between ice, water and steam. Study it and answer the questions that follow
- What name is given to the process represented by energy change ∆H4? (1 mark)
- What is the sign of ∆H3? Give a reason (2 marks)
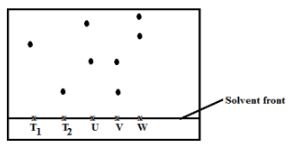
- Samples of urine from three participants U, V and W at an international sport meeting were spotted onto chromatography paper alongside two from illegal drugs T1 and T2. A chromatogramwas run using methanol. The figure below shows the chromatogram.
- Identify the athlete who had used an illegal drug. (1mark)
- Which drug is more soluble in methanol? (1mark)
- Identify a mistake made on the chromatogram. (1mark)
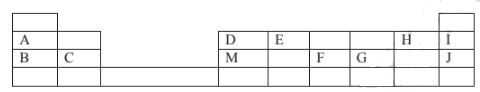
- The grid below is part of the periodic table. Study it and answer the questions that follow. Theletters are not actual symbols of elements.
- What is the name given to the chemical family of element C? (1mark)
- Would element B reacts with J? Explain. (1mark)
- Compare the melting points of B and M. (1mark)
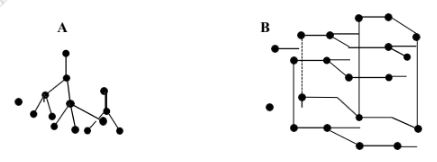
- The following diagrams show the structure of two allotropes of carbon. Study them and answerthe questions that follow.
- Name the allotropes. A and B (1 mark) A:
- Give one use of A. (½ mark)
- Which allotrope conducts electricity? Explain. (1½ marks)
-
- A few drops of freshly prepared Iron (II) Sulphate solution were added to Potassium nitrate solution in a test-tube. Concentrated sulphuric (VI) acid was then carefully added to the mixture. State the observations that were made. (1mark)
- Write an equation for the reaction that occurs when solid potassium nitrate is strongly heated. (1mark)
- What is the role of the test shown in (a) above. (1mark)
-
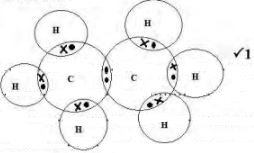
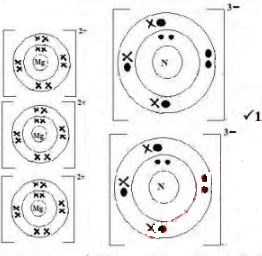
- Using electrons in the outermost energy level, draw the dot (•) and cross (X) diagrams torepresent bonding in:
- C2H6 (1mark)
- Magnesium nitride (1mark)
- The formula of a complex ion is [Cu(NH 3 )4 ]2+ . Name the type of bond that is likely to exist between copper and ammonia in the complex. (1 mark)
- Using electrons in the outermost energy level, draw the dot (•) and cross (X) diagrams torepresent bonding in:
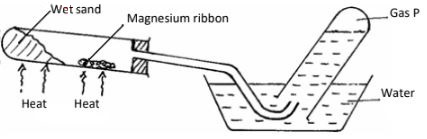
- The set-up below can be used to study the reaction of magnesium and steam
- Name gas P. (1 mark)
- Explain the observation made when copper is used instead of magnesium in the set up above? (1 mark)
- Write the equation for the reaction between magnesium and steam. (1mark)
- 280cm³ of nitrogen gas diffuse through a porous plug in 70 seconds. How long will it take 400cm³ of carbon (IV) oxide gas to diffuse through the same porous plug? (C = 12, O = 16, N = 7).(3mks)
- State two factors that accelerate rusting. (2 marks)
-
- Define the term ionization energy. (1mark)
- State and explain a factor that determine the value of ionization energy of a given element. (2marks)
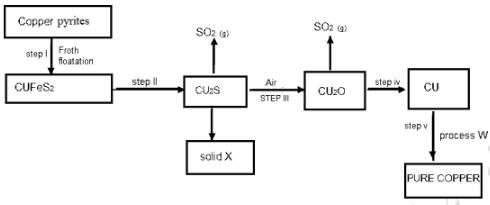
- Study the flow chart below and answer the questions that follow
- Identify
- Solid X (½ mark)
- Process W (½ mark)
- Write an equation for the reaction in step II. (1mark)
- Explain why Copper is suitable in making soldering equipment. (1mark)
- Identify
- The table below shows the solubility of a salt at various temperatures.
Temperature °C Solubility (g/100g water) 0 36 40 30 80 25 100 22 120 20 - Define the term Fractional Crystallization. (1 mark)
- Calculate the mass of salt formed when 20g of a saturated solution of the salt at 0°C is place in a water bath maintained at 100°C. (2 marks)
- The table below shows properties of some elements A,B,C and D which belong to the same period of the periodic table. The letters do not represent the actual symbols of the elements.
Element A B C D M.P.°C 1410 98 -101 660 Atomic radii(nm) 0.117 0.186 0.099 0.143 Electrical conductivity Poor Good Non conductor Good - Arrange the elements in the order they would appear in the period. Give a reason. (2 marks)
- Select the metallic element which is better conductor of electricity. Give a reason. (1 mark)
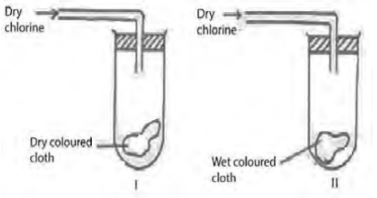
- Study the diagrams below.
- State the observations made at I and II. (1mark)
- Write the equations to show the reaction in II if dry sulphur (IV) oxide was used in place of dry chlorine. (2marks)
- A radioactive substance weighing M kg took 1900 years for the original mass to reduce to 15kg. Given that half-life of the radioactive substance is 380 years;
- Determine the original mass of the radioactive substance. (2 marks)
- State two uses of radioactivity in medicine. (1 mark)
- 20cm³ of a dibasic acid required 25cm³ of 0.1M NaOH for complete neutralization.
- How many moles of sodium hydroxide reacted with the dibasic acid? (1mark)
- Calculate the concentration of the dibasic acid in moles per litre. (2mks)
- The table below shows the standard reduction potentials for four half–cells. Study it and answer the questions that follow (letter are not the actual symbols for the elements)
Eθ (Volts) F2(aq) + 2 e → 2F-(aq)
G2+(aq) + 2e → G(s)
H2+(aq) + 2e → H(s)
2J+(aq) + 2e → J2(g)+0.54
-0.44
+0.34
0.00- Identify the strongest reducing agent. Explain (1mark)
- Write the equation for the reaction which takes place when solid G is added to a solution containing H2+ ions. (1 mark)
- Calculate the E0 value for the reaction in (ii) above. (1mark)
- Starting with solid lead (II) carbonate, briefly describe how a sample of lead (II) chloride can be prepared. (3marks)
- Chlorine and iodine are elements in the same group in the periodic table.
- What observation would be made if chlorine gas is bubbled through aqueous sodium iodine? Explain using an ionic equation. (2 marks)
- Using the equation in (a) above, identify and explain the reducing agent. (1 mark)
- Using energy cycle diagram, calculate the enthalpy of formation of ethane from the information given below.
C(s) + O2(g) → CO2(g) ∆HC = -394kJmol-1
H2(g) + O2(g) → H2O(l) ∆HC = -286kJmol-1
C2H6(g) + 5O2(g) → 2CO2(g) + 3H2O(l) ∆Hc = -1300kJmol-1 (3 marks) -
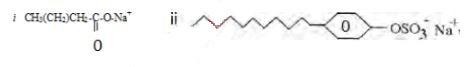
- Identify the following cleansing agents. (2 mark)
- State one disadvantage of using the cleansing agent in (a) (ii) above. (1mark)
- Identify the following cleansing agents. (2 mark)
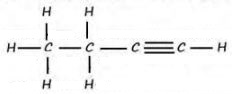
- The empirical formula of a hydrocarbon is C2H3. The hydrocarbon has a relative molecular mass of 54. (H=1.0, C=12.0).
- Determine the molecular formula of the hydrocarbon. (1mark)
- Draw the structural formula of the hydrocarbon. (1mark)
- To which homologous series does the hydrocarbon drawn in (b) above belong? (1mark)
-
- State the Boyle’s law. (1mark)
- A gas occupies 300cm3 at 23oC and 100,000 Pa. What will be its volume at 00C and 101325Pa? (2marks)
- In the laboratory, hydrogen sulphide gas is prepared by action of dilute hydrochloric acid on metal sulphides.
- Name the metal sulphide that can be used in preparing the gas. (1mark)
- Write down the equation for the reaction in (a) above. (1mark)
- Give one chemical test for hydrogen sulphide gas. (1mark)
- Some crystals of sugar cane were placed in a test-tube and a few drops of concentrated sulphuric (VI) acid added to it.
- State what was observed. (1mark)
- What name is given to the property of concentrated sulphuric (VI) acid in (i) above? (1mark)
- Write an equation for the reaction between glucose, C6H12O6 and H2SO4 (l). (1mark)
- The following equation shows a reversible reaction.
H2(g) + Br2(g) ⇌ 2HBr(g ) ∆H = − 74.4kJ
brown colourless- State and explain the observation that can be made when:-
- Temperature is increased. (1½marks)
- Pressure is reduced (1½marks)
- State and explain the observation that can be made when:-

Marking Scheme
-
- Soot it produces dirtifies the apparatus
The flame is not very hot - Opening the air hole fully
- Soot it produces dirtifies the apparatus
-
- Latent heat of fusion
- Negative(-)
The process looses heat
- Latent heat of fusion
-
- V
- T1
- Solvent front is labelled in place of baseline
- V
-
- Alkaline earth metals
- No. J does not form compounds as it is chemically stable already
- M has a higher melting point than B as it has a strong metallic bond or it has more delocalised electrons
- Alkaline earth metals
-
-
- Diamond
- Graphite
- Jewellery/ Making drilling machines/ bits
- B. Existence of delocalised electrons which transfer electricity
-
-
- A brown ring/ substance is formed between potassium nitrate and concentrated sulphuric (VI) acid
- 2KNO3(s) → 2KNO2(s) + O2(g)
- Test for nitrates
- A brown ring/ substance is formed between potassium nitrate and concentrated sulphuric (VI) acid
-
-
-
- Dative covalent bond
-
-
- Hydrogen
- Copper would not react with steam copper is below hydrogen in the reactivity series and hence cannot displace it in a reaction
- Mg(s) + H2O(q) → MgO(s) + H2(g)
- Hydrogen
- RN2 = 280/70 = 4cm3/sec
RCO2 = 400/t
Hence 4/400/t = √44/28
t= √44/28 x 100
= 125.36 sec - Low pH/ acidic conditions
High temperature
Salty conditions -
- The minimum amount of energy needed to remove an electron from an atom in its gaseous state.
- Atomic radius/ size of the atom
The distance between the outermost electron and the nucleus affects nuclear attraction
- The minimum amount of energy needed to remove an electron from an atom in its gaseous state.
-
-
- Iron (II) oxide/ FeO
- Electrolysis/ Purification
- Iron (II) oxide/ FeO
- 2CuFeS2(s) + 4O2(g) → Cu2S(s) + 2FeO(s) + 3SO2(g)
- This is due to its high thermal conductivity
-
-
- A process by which solutes are separated from each other according to their solubilities/ separation of salts by making use of the differences in their solubility.
- From the table, 100g of saturated solution will give= 36g - 22g = 14g
Hence , if 100g of saturated solution = 14g of salt
Therefore 20g of saturated solution
= 20 x 14
100
= 2.8g of salt
- A process by which solutes are separated from each other according to their solubilities/ separation of salts by making use of the differences in their solubility.
-
- BDAC
Because the atomic radius decreases across a period/ decrease with increase in nuclear charge or number of proton - D
Becauseit has more delocalised electrons than B
- BDAC
-
- In I - There is no bleaching
In II - There is bleaching - SO2(g) + H2O(l) → 2H+(aq) + SO2-3(aq)
SO2-3(aq) + Dye → SO2-4(aq) + (Dye - [O])
- In I - There is no bleaching
-
- Number of half-life = 1900/380 = 5
480kg ← 240kg ← 120kg ← 15kg
Hence M= 480kg - Used to destroy cancerous cells
Used to sterilise surgical instruments
- Number of half-life = 1900/380 = 5
-
- Moles of NaOH = 25/1000
= 0.0025 moles - H2X(aq) + NaOH(aq) → Na2X(aq) + 2H2O(l)
Mole ratio Acid:NaOH= 1:2 (either from equation or just stated)
Moles of H2X= 1/2 x 0.0025 = 0.00125
Concentration in moles per litre
= 0.00125 x 1000
20
= 0.0625 moles per litre
- Moles of NaOH = 25/1000
-
-
- G(s) This is because it has the highest negative electrode potential
- G(s) + H2+(aq) → G2+(aq) + H(s)
- +0.34 + 0.44 = +0.78v
- G(s) This is because it has the highest negative electrode potential
-
-
- To excess lead(II) carbonate, add nitric (V) acid
- Filter to obtain lead(II) nitrate filtrate
- Add dilute hydrochloric acid/ any soluble chloride tot he filtrate
- Filtrate to obtain lead (II) chloride residue
-
-
- Colourless solution would turn brown
- This is because chlorine displaces iodine from iodine solution
Cl2(g) + 2I-(aq) → 2Cl-(aq) + I2(aq)
- I-(aq)
The oxidation number of iodine increases from in -1 in I-(aq) to 0 in I2
-
-
-
-
- Soapy detrgent
- Soapless detergent
- Non biodegradable hence pollutes the environment
It is expensive
-
-
- (C2H3)n = 54
27n = 54
n=2
Molecular formula is C4H6 -
- Alkyne
- (C2H3)n = 54
-
- The volume of a fixed mass of a gas is inversely proportional to its pressure at constant temperature
- v1 = 300cm3
T1= 23 + 273 = 296K
P1= 100,000Pa
V2 = x
T2 = 0 + 273 = 273K
P2= 101, 325Pa
V2 = 100,000 x 300 x 273
296 x 101,325
= 273.07cm3
- The volume of a fixed mass of a gas is inversely proportional to its pressure at constant temperature
-
- Iron(II) sulphide
(accept metal chloride that do not form insoulble chlorides) - FeS(s) + 2HCl(aq) → FeCl2(aq) + H2S(g)
- Use lead acetate paper or lead(II) ethanoate ppaer or soak a paper in lead (II) nitrate solution. The paper turns from white to black.
- Iron(II) sulphide
-
- The white crystals of sugar can changes into a black mass
- Dehydration property
- C6H12O6(g) + H2SO4(l) → 6C(s) + 6H2O(l)
- The white crystals of sugar can changes into a black mass
-
-
- Intensity of the brown colour will increase. More recatants will be formed as the forward reaction is exothermic
- No effect. The volume of the reactants and products are equal.
- Intensity of the brown colour will increase. More recatants will be formed as the forward reaction is exothermic
-
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 1 Questions and Answers - Maranda Mocks 2021/2022 Exams.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students