QUESTIONS
- The grid below shows a section of the periodic table. The letters do not represent the actual symbols of the elements
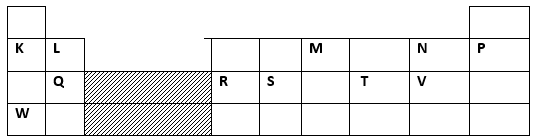
- Name the family into which element P belongs to ( 1mk)
- Which two elements form the most stable carbonates ( 2mks)
- With a reason ,identify elements in period 3 with largest atomic radius ( 2mks)
- Write the formula of the compound formed between Q and M ( 1mk)
- State two uses of element R and for each use , state property of element R that makes its possible for the use
- Use ( 1mk)
Property (1 mk) - Use ( 1mk)
Property (1mk)
- Use ( 1mk)
- Using dots (.) and cross (X), show bonding in the compound formed between R and oxygen ( 2 mks)
- In terms of structure and bonding explain why the oxides of element T has relatively low boiling points ( 2mks)
- The diagram below shows the set-up of the apparatus used by a student to determine the enthalpy change of combustion of ethanol. The heat produced by burning fuel warms a known mass of water.
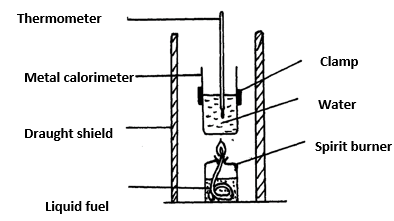
Results
Volume of water in the beaker = 500 cm3
Initial temperature of water= 12ºC
Final temperature of water = 31.5ºC
Mass of ethanol burnt = 1.50g
Density of water = 1 g/cm3
Specific heat capacity = 4.2 Jg-1K-1- Define molar heat of combustion. (1 MK)
-
- Calculate the heat required to raise the temperature of the water from 12ºC to 31.5ºC. (2 mks)
- Find the molar enthalpy of combustion of ethanol. (2 mks)
(C=12,H=1,O=16
- An accurate value for ΔHC of ethanol is -1368 kJmol-1. State two sources of errors for the low figure obtained. (2 mks)
- Draw an energy level diagram for the combustion of ethanol. (2 mks)
- State one factor that one may consider when choosing kerosene as a fuel in Eldoret town. (1 mk)
-
- Name the following compounds ( 3mks)
- CH3CH2CH2COOH
- H2C Br– CH(CH3) – CH2 – CBr = CH – CH3
- CH3CH2COOCH2CH3
- two types of detergents P and Q can be represented as
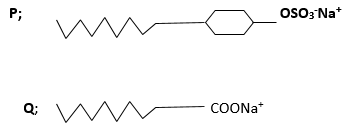
- Identify each type of the detergent ( 2mks)
P………………………………………………………………………………………………………
Q…………………………………………………………………………………………………….. - Which of the two detergent is the best to use with hard water ,give reason ( 2mks)
- State one disadvantage of detergent P ( 1mk)
- State advantage of detergent Q ( 1mk)
- Identify each type of the detergent ( 2mks)
- an hydrocarbon can be represented as follows
CH2=CH2- Name the hydrocarbon ( 1mk)
- Name two reagents that can be reacted together to generate the hydrocarbon ( 2mks)
- Name the following compounds ( 3mks)
-
- Sulphur is extracted from underground deposits by a process in which three concentric pipes are sunk down to the deposits as shown below
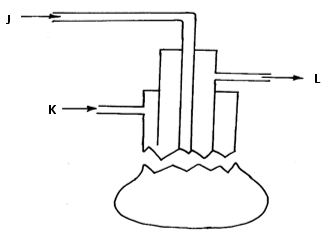
- Name the above process used to obtain sulphur from the underground deposits ( 1 mk)
- Name the substance passed through pipe (2mks)
A………………………………………………………………………………………………………………………………………………
B……………………………………………………………………………………………………………………………………………….. - State two properties of sulphur that makes it possible to extract using the above process ( 2 mks)
- The diagram below shows the contact process used in the manufacture of concentrated sulphuric (VI) acid
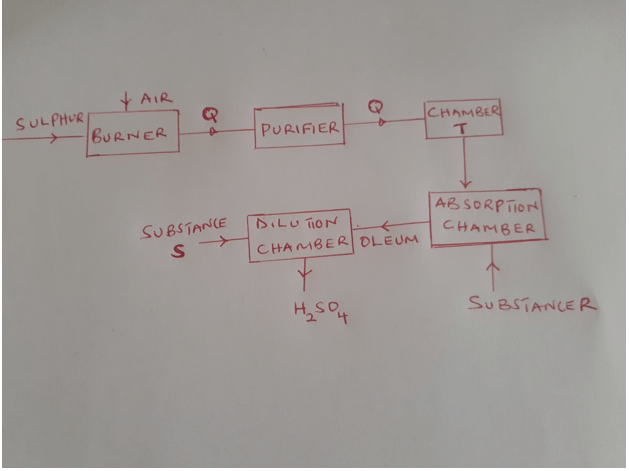
- Identify the following substance; ( 4 mks)
- Q formed in the burner
- Formed in Chamber T
- R
- S
- Write the chemical equation occurring in the dilution chamber ( 1 mk)
- Why is it necessary to pass substance g through a purifier ( 1mk)
- State one use of sulphuric ( VI) acid ( 1 mk)
- Identify the following substance; ( 4 mks)
- Sulphur is extracted from underground deposits by a process in which three concentric pipes are sunk down to the deposits as shown below
-
- Define the term electrolysis ( 1mk)
- State two function of the salt bridge during electrolysis ( 2mks)
- The reduction potential of elements K,L,M and P are as given below
Half reaction
E0 (volts)
K+ + e - → K (s)
-1.46
L2+ + 2e- → L(s)
+0.49
P+ + e- → P(s)
-0.86
M2+ 2e- → M(s)
-2.69
N+ + e- → N(s)
+0.52
- Which letter represents the, strongest reducing agent , give reason ( 2mks)
- Which two letters represents elements whose half cell would form an electrochemical cell with largest e.m.f ( 1 mk)
- Calculate the e.m.f of the cell formed in (ii) above ( 2mks)
- During the electrolysis of a molten chloride of metal q , a current of 0.25A was passed through the molten chloride for 2 hours and 10 minute .Given that 0.9 g of metal Q were deposited at the cathode
- Calculate the quantity of electricity passed ( 1mk)
- Charge carried by the ions of metal Q given that R.A.M of metal Q is 84 (3 mks)
- Ammonia can be prepared in the lab by reaction of Calcium hydroxide and an ammonium salt.
- Write an equation for the reaction that will take place. (1 mk)
- Calculate the volume of ammonia produced at room temperature and pressure given that 20g of calcium hydroxide reacted fully. (Ca = 40,H = 1,O = 16,N = 14,MGV = 24dm3) (2 mks)
-
- Write an equation to show how ammonia is used to make phosphate fertilizer. (1 mk)
- Determine the percentage by mass of Nitrogen in the above fertilizer. (N = 14,H = 1,P = 31,O = 16) (1 mk)
- State the importance of using ammonium phosphate over urea as a fertilizer (1 mk)
- Describe how the presence of nitrate ions can be determined in a solution using concentrated Sulphuric (VI) acid as one of the reagents. (3 mks)
- State one danger of continued use of Nitrogenous fertilizers. (1 mk)
- 1g of magnesium ribbon was reacted with hydrochloric acid at room temperature in order to investigate how the rate of reaction varies with time. The results obtained were recorded as shown below.
Time (seconds)
0
20
40
60
80
100
120
140
160
180
Volume of gas produced (cm3)
0
10
20
26
32
35
38
39
40
40
-
- On the graph provided, plot a graph of volume of gas produced against time taken. Label the graph K. (3 Mrk)
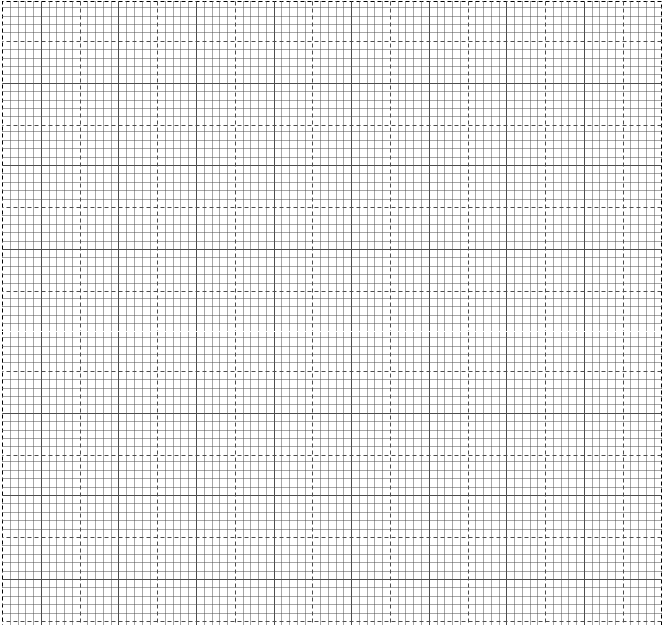
- From the graph determine the rate of production of the gas at 110 seconds. (2 Mks)
- On the graph provided, plot a graph of volume of gas produced against time taken. Label the graph K. (3 Mrk)
- On the same axis sketch the graph you would expect to obtain if:-
- The same mass of powdered magnesium was used instead of magnesium ribbon. Label the graph Y. (1 Mk)
- If the temperature of the solution mixture was reduced from 250C to 150C. Label the graph Z. (1 Mk)
- Determine the mass of magnesium ribbon that remained unreated in this experiment (Mg = 24, Molar gas volume = 24dm3 at r.t.p) (3 Mks)
-
Download Chemistry P2 Questions - Momaliche 4 cycle Post Mock Exams 2021/2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

