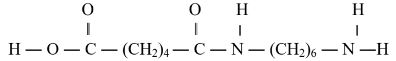CHEMISTRY
PAPER 1
THEORY
INSTRUCTIONS TO CANDIDATES
- Write your name and index number in the spaces provided above.
- Answer all the questions in the spaces provided.
- All working must be clearly shown.
- Non-programmable silent electronic calculators and KNEC mathematical tables may be used.

Questions
- The pH values of some solutions labeled E to I are given in the table below. Use the information to answer the questions that follow.
pH 14.0 1.0 9.0 6.5 5.0 Solution E F G H I - Identify the solution with the highest concentration of hydroxide ions. Explain (1mk)
- Which solution can be used as a remedy for acid indigestion in the stomach? Explain (1mk)
- Which solution would react explosively with Potassium metal? (1mk)
-
- Distinguish between ionization energy and electron affinity (2mks)
- The table below shows first ionization energies of metals represented by letters A, B, C and D. The metals are in the same group of the periodic table.
Which of the metals has the smallest atomic radius? Explain. (2mks)Metal A B C D 1st ionization energy (kJ/mole) 402 496 520 419
- An element:
- To which chemical family does it belong? (1/2mk)
- Write the electron arrangement of the atom. ( 1 /2mk)
- Draw the structure of its ion. (1mk)
-
- Define electrolysis. (1mk)
- During the electrolysis of molten aluminium oxide, write the equations at the;
Anode -..................................................................................... (1mk)
Cathode -..................................................................................... (1mk)
- In an experiment to determine the percentage purity of Sodium carbonate produced in the Solvay process, 2.15g of the sample reacted with exactly 40.0cm3 of 0.5M Sulphuric (VI) acid. Determine the percentage purity of sodium carbonate in the sample. (3mks)
- Y is a product of gaseous reaction which results in an equilibrium mixture being formed.
Reactants ⇌ Y
The percentage of Y in equilibrium at various temperatures and pressure is shown in the following table.
Use this data to deduce, giving a reason for each case;Temperature (ºC) 1 atm 100 atm 200 atm 550
650
750
8500.77
0.032
0.016
0.096.70
3.02
1.54
0.8711.9
5.71
2.99
1.68- Whether production of Y is exothermic or endothermic. (2mks)
- Whether production of Y involves an increase or a decrease in number of moles of gas present. (2mks)
- State and explain what is observed when moist red flowers are dropped in a gas jar containing Sulphur (IV) oxide gas. (3mks)
- A sample of water collected from River Gucha is suspected to contain sulphate ions. Describe an experiment that can be carried out to determine the presence of the sulphate ions. (2mks)
- During distillation in a laboratory the distillate can be collected either by a beaker or a conical flask.
- Define the term distillate. (1mk)
- Explain why a conical flask is the most preferred apparatus for the collection of the distillate. (1mk)
- Draw the diagram of a graduated conical flask. (1mk)
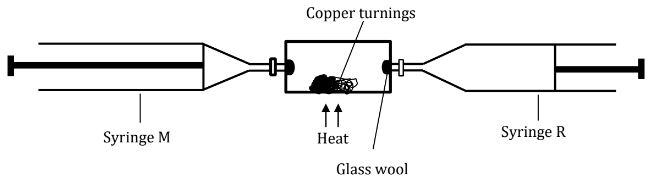
- In an experiment to determine the proportion of oxygen in air, copper turnings were packed in excess in a long combustion tube connected to two syringes of 110cm3 each in volume. At the beginning of the experiment, syringe R contained 110cm3 of air while syringe M was closed and empty as shown.
Air was passed over the heated copper slowly and repeatedly until there was no further change in volume. 97.5cm3 of air remained in syringe M.- State and explain the observation made in the combustion tube. (2mks)
- If the volume of air in the combustion tube at the beginning of the experiment was 23.8cm3 and at the end of the experiment reduced to 10cm3, calculate the percentage of the active part of air. (2mks)
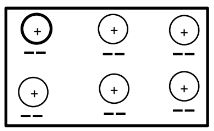
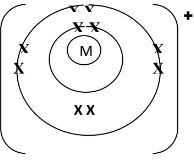
- Below is a structure of an element X. Use it to answer the questions that follow.
- Name the chemical family to which element X belongs. Give a reason. (2mks)
-
- Define covalent bond. (1mk)
- Using dots ( .) of cross ( x ) diagram, show bonding in Carbon (II) Oxide. (1mk)
-
-
- State two allotropes of Carbon. (1mk)
- Explain the differences in their densities. (2mks)
-
- Name the process used for large scale production of Sodium Carbonate using brine as raw material. (1mk)
- Write the overall chemical equation for the reaction in the carbonator. (1mk)
- Name two gases recycled in the above process (1mk)
-
- Name the following compounds using the IUPAC system. (3mks)
-
- CH3CH2OH
-
- Describe how to prepare Methane gas starting with soda lime (3mks)
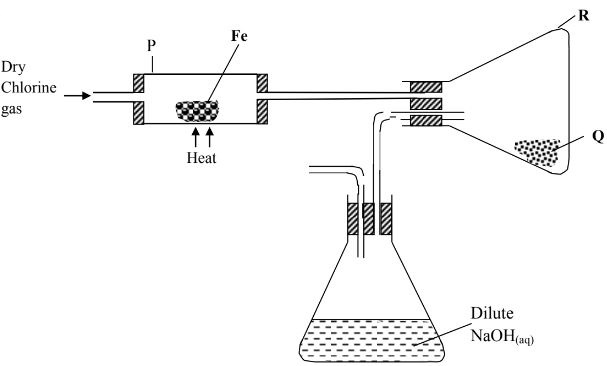
- The diagram below shows how chlorine reacts with metals in the laboratory. Study it and answer the questions that follow.
- Name substance Q. (1mk)
- Give a reason why substance Q is not collected in the combustion tube P. (1mk)
- Write chemical equation for the reaction that occurs in the conical flask containing Sodium hydroxide. (1mk)
-
- Water sample is found to contain Mg2+,Cl-, SO42-, and Ca2+. Identify the type of water hardness (1mk)
- Which type of detergent is more suitable with the water sample above. Give a reason (2mks)
- Permanent water hardness cannot be removed by boiling. Explain (1mk)
- Starting with lead metal, write procedure on preparation of lead(II) nitrate crystals (3mks)
- The following chemical equations show the effects of heat on nitrates.
2B(NO3)2(s) → 2BO(s) + 4NO2(g) + O2(g)
2ANO3(s) → 2ANO2(s) + O2(g)
2CNO3(s) → 2C(s) +2NO2(s) + O2(g)- Arrange elements A, B and C from the most reactive to the least reactive. (11/2mks)
- Give one example of element A, B and C. (11/2mks)
- Copper (II) sulphate crystals, a boiling tube, a test-tube, a beaker and other necessary requirements were used in an experiment to determine the type of change that occurred when the crystals were heated.
- Draw a labelled diagram to represent the set-up at the end of the first part of the experiment. (3mks)
- After the second part of the experiment was done, state the conclusion that was made about the type of change undergone by copper (II) sulphate crystals when heated. (1mk)
-
- Distinguish between chromatography and a chromatogram. (1mk)
- State the role of chromatography in the administration of international athletics competitions. (1mk)
- Study the polymer shown below.
- Name the polymer. (1mk)
- Identify two monomers that make up the polymer. (2mks)
- Give one use of the polymer (1mark)
-
- State Charles law. (1mk)
- A gas occupies 450cm3 at 27ºC. What volume would the gas occupy at 177ºC if its pressure remains constant? (2mks)
- A colourless liquid was suspected to be water. State two ways to confirm.
- Purity of the water. (1mk)
- That the liquid was water. (2mks)
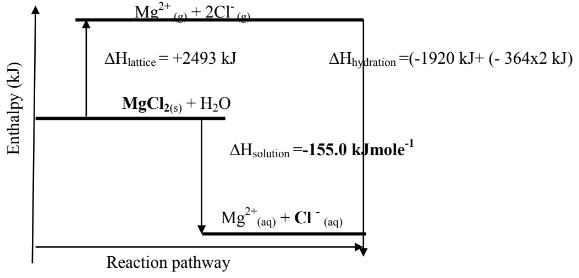
- Use the following information to answer the questions that follow
ΔH lattice MgCl2 = +2493 kJ/ mol
ΔH hydration Mg2+ = - 1920 kJ/ mol
ΔH hydration Cl - = -364 kJ/ mol- Calculate the heat of solution of magnesium chloride. (2mks)
- Draw an energy level diagram for the dissolving of magnesium chloride (2mks)
-
- A solution of aqueous sodium hydroxide is added to a gas jar of nitrogen (IV) oxide and shaken.
State and explain the observation made (2mks) - Write the chemical equation for the reaction above (1mk)
- A solution of aqueous sodium hydroxide is added to a gas jar of nitrogen (IV) oxide and shaken.

Marking Scheme
- The pH values of some solutions labeled E to I are given in the table below. Use the information to answer the questions that follow.
pH 14.0 1.0 9.0 6.5 5.0 Solution E F G H I - Identify the solution with the highest concentration of hydroxide ions. Explain (1 mark)
- E, Strong base
- E, Strong base
- Which solution can be used as a remedy for acid indigestion in the stomach? Explain (1 mark)
- G, Magnesium Hydroxide
- G, Magnesium Hydroxide
- Which solution would react Explosively with Potassium metal? (1 mark)
- F, Dilute acids reacts with Alkali Metals Explosively/strong acid
- Identify the solution with the highest concentration of hydroxide ions. Explain (1 mark)
-
- Distinguish between ionization energy and electron affinity (2mk)
- Ionization energy is the energy required to lose/donate an electron in an atom of an element in its gaseous state while electron affinity is the energy required to gain/acquire extra electron by an atom of an element in its gaseous state.
Electron affinity is the energy required to gain an electron in an atom of an element in
gaseous state.
- Ionization energy is the energy required to lose/donate an electron in an atom of an element in its gaseous state while electron affinity is the energy required to gain/acquire extra electron by an atom of an element in its gaseous state.
- The table below shows first ionization energies of metals represented by letters A, B, C and D. The metals are in the same group of the periodic table.
Which of the metals has the largest atomic radius? Explain. (2mks)- C, has the smallest atomic radius, hence stronger nuclear force of attraction holding the outermost electron
- C, has the smallest atomic radius, hence stronger nuclear force of attraction holding the outermost electron
- Distinguish between ionization energy and electron affinity (2mk)
- An element is represented as :2311M
- To which chemical family does it belong? (1/2mark)
- Alkali metals
- Alkali metals
- Write the electron arrangement of the atom .(1/2mark)
- 2.8.1
- 2.8.1
- Draw the structure of its ion. (1mark)
- To which chemical family does it belong? (1/2mark)
-
- Define electrolysis. (1 mark)
- Process by which an electrolyte gets decomposed when an electric current is passed through it.
- Process by which an electrolyte gets decomposed when an electric current is passed through it.
- During the electrolysis of molten aluminium oxide, write the equations at the;
- Anode - 6O2-(l) → 3O2(g)+12e (1 mark)
Cathode 4Al3+(l) +12e → 4Al(l) (1 mark)
- Anode - 6O2-(l) → 3O2(g)+12e (1 mark)
- Define electrolysis. (1 mark)
- In an experiment to determine the % purity of Sodium carbonate produced in the Solvay process ,2.15g of the sample reacted with exactly 40.0cm3 of 0.5M Sulphuric(VI)acid. determine the % purity of sodium carbonate in the sample.
- Na2CO3(aq) + H2SO4(aq) → Na2SO4(aq) + CO2(g) + H2O(l)
Mole ratio Na2CO3 :H2SO4 ⇒ 1:1
Moles H2SO4 = Molarity x Volume
1000
0.5 x 40.0 = 0.02 Moles
1000
Moles of Na2CO3 = 0.02 Moles
Molar mass of Na2CO3 = 106g
Mass of Na2CO3 = moles x Molar mass⇒ 0.02 x 106 = 2.12 g
% of Na2CO3=( 2.12 g x 100) = 98.6047%
2.15
- Na2CO3(aq) + H2SO4(aq) → Na2SO4(aq) + CO2(g) + H2O(l)
- Y is a product of gaseous reaction which results in an equilibrium mixture being formed.
Reactants Y
The percentage of Y in equilibrium at various temperatures and pressure is shown in the following table.
Use this data to deduce, giving a reason for each case;- Whether production of Y is exothermic or endothermic. (2 marks)
- Exothermic. An increase in temperature reduces the yield of Y; favours the backward endothermic reaction
- Exothermic. An increase in temperature reduces the yield of Y; favours the backward endothermic reaction
- Whether production of Y involves an increase or a decrease in number of moles of gas present. (2 marks)
- Decrease in the number of moles. Increase in pressure increases the yield of Y; favours forward reaction that reduces moles/volume/molecules of gas present
- Decrease in the number of moles. Increase in pressure increases the yield of Y; favours forward reaction that reduces moles/volume/molecules of gas present
- Whether production of Y is exothermic or endothermic. (2 marks)
- State and explain what is observed when moist red flowers are dropped in a gas jar containing Sulphur (IV) oxide. (2marks)
- When moist red flowers are dropped into a gas jar containing sulphur(IV) oxide, the flowers are bleached/turn white. Sulphur(IV) oxide combines with moisture, forming sulphuric(IV) acid which combines with oxygen from the dye to form sulphuric(VI) acid. When the dye loses oxygen it becomes colourless/white/bleached, the dye undergoes reduction while the sulphuric(IV) acid is oxidised.
SO2(g) + H2O(l) → H2SO3(aq)
H2SO3(aq) + Dye → H2SO4(aq) + Colourless material.
- When moist red flowers are dropped into a gas jar containing sulphur(IV) oxide, the flowers are bleached/turn white. Sulphur(IV) oxide combines with moisture, forming sulphuric(IV) acid which combines with oxygen from the dye to form sulphuric(VI) acid. When the dye loses oxygen it becomes colourless/white/bleached, the dye undergoes reduction while the sulphuric(IV) acid is oxidised.
- A sample of water from River Gucha is suspected to contain sulphate ions. Describe an experiment that can be carried out to determine the presence of the sulphate ions.(3 marks)
- Add from Pb(NO3)2(aq)/Ba(NO3)2(aq); followed by acidify with dilute HNO3(aq) A white precipitate which persist on addition of the acid is formed; showing presence of SO42- ion;
- Add from Pb(NO3)2(aq)/Ba(NO3)2(aq); followed by acidify with dilute HNO3(aq) A white precipitate which persist on addition of the acid is formed; showing presence of SO42- ion;
- During distillation in a laboratory the distillate can be collected either by a beaker or a conical flask.
- Define the term distillate. (1 mark)
- condensed liquid collects in the receiver
- condensed liquid collects in the receiver
- Explain why a conical flask is the most preferred apparatus for the collection of the distillate. (1 mark)
- The narrow mouth ensures no spillage.
- The narrow mouth ensures no spillage.
- Draw the diagram of a graduated conical flask. (1 mark)
- Define the term distillate. (1 mark)
- In an experiment to determine the proportion of oxygen in air, copper turnings were packed in excess in a long combustion tube connected to two syringes of 110cm3 each in volume. At the beginning of the experiment, syringe R contained 110cm3 of air while syringe M was closed and empty as shown.
Air was passed over the heated copper slowly and repeatedly until there was no further change in volume. 97.5cm3 of air remained in syringe M.- State and explain the observation made in the combustion tube. (2 marks)
- A black solid due to the formation of copper (ii) oxide
- A black solid due to the formation of copper (ii) oxide
- If the volume of air in the combustion tube at the beginning of the experiment was 23.8cm3 and at the end of the experiment reduced to 10cm3, calculate the percentage of the active part of air. (2 marks)
- 0.579831 x 100 = 57.9831%
- 0.579831 x 100 = 57.9831%
- State and explain the observation made in the combustion tube. (2 marks)
- Below is a structure of an element X. Use it to answer the questions that follow.
- Name the chemical family to which element X belongs. Give a reason. (2 marks)
- Alkaline- Earth metal. Has two valence electrons
- Alkaline- Earth metal. Has two valence electrons
-
- Define covalent bond. (1 mark)
- Bond which involves sharing of electrons contributed by both atoms
- Using dots ( ) of cross ( x ) diagram, show bonding in Carbon (II) Oxide.(1 mark)
- Define covalent bond. (1 mark)
- Name the chemical family to which element X belongs. Give a reason. (2 marks)
-
-
- State two crystalline allotropes of Carbon. (1mark)
- Carbon-diamond
- Carbon-graphite
- Explain the differences their densities. (2 marks)
- Diamond has very high density than graphite because; it has a very closely packed giant tetrahedral structure joined by strong covalent bonds
- State two crystalline allotropes of Carbon. (1mark)
-
- Name the process used for large scale production of Sodium Carbonate using brine as raw material. (1 mark)
- Solvay Process
- Solvay Process
- Write the overall chemical equation for the reaction in the carbonator. (1 mark)
- CO2(g) + H2O(l) + NaCl(aq) + NH3(g) → NaHCO3(s) + NH4Cl(aq)
- CO2(g) + H2O(l) + NaCl(aq) + NH3(g) → NaHCO3(s) + NH4Cl(aq)
- Name two gases recycled in the above process (1 mark)
- Ammonia gas , Carbon(IV)Oxide
- Ammonia gas , Carbon(IV)Oxide
- Name the process used for large scale production of Sodium Carbonate using brine as raw material. (1 mark)
-
- Name the following compounds using the IUPAC system. (3 marks)
-
- 3-bromohept-2-ene
- 3-bromohept-2-ene
- CH3CH2OH
- Ethan-1-ol/ethanol
- Ethan-1-ol/ethanol
-
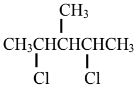
- 2,4-dicloro-3-methylpentane
- 2,4-dicloro-3-methylpentane
-
- Describe how to prepare Methane gas starting with soda lime (3marks)
- Sodium ethanoate and an equal mass of soda lime is put in a hard glass test tube, upon mixing them thoroughly in a mortar. The mixture is heated thoroughly in the test-tube. A colorless gas collects over water/syringe
- Sodium ethanoate and an equal mass of soda lime is put in a hard glass test tube, upon mixing them thoroughly in a mortar. The mixture is heated thoroughly in the test-tube. A colorless gas collects over water/syringe
- The diagram below shows how chlorine reacts with metals in the laboratory. Study it and answer the questions that follow.
- Name substance Q. (1 mark)
- Iron (III) chloride
- Iron (III) chloride
- Give a reason why substance Q is not collected in the combustion tube P.(1 mark)
- Iron (III) chloride sublimes on heating; the black solid changes to red-brown fumes on heating.
- Iron (III) chloride sublimes on heating; the black solid changes to red-brown fumes on heating.
- Write chemical equation for the reaction that occurs in the conical flask containing Sodium hydroxide. (1 mark)
- Cl2(g) + 2NaOH(l) → NaCl(aq) + NaClO(aq) + H2O(l)
- Cl2(g) + 2NaOH(l) → NaCl(aq) + NaClO(aq) + H2O(l)
- Name substance Q. (1 mark)
-
- Water sample is found to contain Mg2+,Cl-,SO42-, and Ca2+. Identify the type of waterhardness (1mks)
- Permanent water hardness
- Permanent water hardness
- Which type of detergent is more suitable with the water sample above. Give a reason(2marks)
- Soapless Detergent, does not form scum with water
- Soapless Detergent, does not form scum with water
- Permanent water hardness cannot be removed by boiling. Explain (1mks)
- The soluble sulphates and chlorides of Mg and Ca do not decompose upon boilin hence cannot be precipitated out.
- The soluble sulphates and chlorides of Mg and Ca do not decompose upon boilin hence cannot be precipitated out.
- Water sample is found to contain Mg2+,Cl-,SO42-, and Ca2+. Identify the type of waterhardness (1mks)
- Starting with lead metal, write procedure on preparation of lead(II) nitrate crystals (3mks)
- Measure dilute Nitric(V) acid and transfer it into a beaker.
- Add Lead powder a little at a time as you stir with a glass rod. Continue adding lead powder until it is in excess.
- Filter the solution and pour the filtrate into an evaporating basin.
- Heat to Evaporate the filtrate to saturation
- Allow the now saturated solution to cool .
- The following chemical equations show the effects of heat on nitrates.
2B(NO3)2(s) → 2BO(s) + 4NO2(g) + O2(g)
2ANO3(s) → 2ANO2(s) + O2(g)
2CNO3(s) → 2C(s) +2NO2(s) + O2(g)- Arrange elements A, B and C from the most reactive to the least reactive. (11/2mks)
- A,B,C
- A,B,C
- Give one example of element A, B and C. (11/2mks)
- A Sodium/Potassium
- B Magnesium/Zinc/Lead/Iron/Copper
- C Silver/Mercury
- Arrange elements A, B and C from the most reactive to the least reactive. (11/2mks)
- Copper (II) sulphate crystals, a boiling tube, a test-tube, a beaker and other necessary requirements were used in an experiment to determine the type of change that occurred when the crystals were heated.
- Draw a labelled diagram to represent the set-up at the end of the first part of the experiment. (3mks)
- After the second part of the experiment was done, state the conclusion that was made about the type of change undergone by copper (II) sulphate crystals when heated. (1mks)
- Chemical change
- Chemical change
- Draw a labelled diagram to represent the set-up at the end of the first part of the experiment. (3mks)
-
- Distinguish between chromatography and a chromatogram. (1mk)
- Chromatography is a method of separating components of a solution mixture by passing it through a medium where the different components move at different rates while
- Chromatogram is the medium through which the solution mixture is passed
- State the role of chromatography in the administration of international athletics competitions. (1mk)
- To test for the presence of illegal drugs
- To test for the presence of illegal drugs
- Distinguish between chromatography and a chromatogram. (1mk)
- Study the polymer shown below.
- Name the polymer. (1mk)
- Nylon 6,6
- Nylon 6,6
- Identify two monomers that make up the polymer. (2 mks)
- hexan-1,6-dioyl dichloride
- hexan-1,6-diamine.
- Give one use of the polymer (1mark)
- to make clothes, plastic ropes and carpets.
- to make clothes, plastic ropes and carpets.
- Name the polymer. (1mk)
-
- State Charles law. (1mk)
- Volume of a given mass of gas is directly proportional to absolute temperature at constant pressure
- Volume of a given mass of gas is directly proportional to absolute temperature at constant pressure
- A gas occupies 450cm3 at 27ºC. What volume would the gas occupy at 177ºC if its pressure remains constant? (2mks)
- 450 x 450 =202,500
202,500 ÷300 = 675cm3
- 450 x 450 =202,500
- State Charles law. (1mk)
- A colourless liquid was suspected to be water. State two ways to confirm.
- Purity of the water. (1mk)
- boils at 100ºc/ freezes at 0ºc
- boils at 100ºc/ freezes at 0ºc
- That the liquid was water. (2mks)
- either use anhydrous copper (ii) sulphate which changes from white to blue or anhydrous cobalt (ii) chloride it changes from blue to pink.
- either use anhydrous copper (ii) sulphate which changes from white to blue or anhydrous cobalt (ii) chloride it changes from blue to pink.
- Purity of the water. (1mk)
- Use the following information to answer the questions that follow
Δ H lattice MgCl2 = +2493 kJ/ mol -1
ΔH hydration Mg2+ = - 1920 kJ/ mol
ΔH hydration Cl - = -364 kJ/ mol- Calculate the heat of solution of magnesium chloride. (2mks)
- MgCl2 --breaking the crystal into free ions → Mg2+(g) + 2Cl-(g) ∆Hl = +2493 kJ
Hydrating the ions;
Mg2+(g) + aq→Mg2+(aq) ∆Hh = - 1920 kJ
2Cl-(g) + aq →2Cl-(aq) ∆Hh = (- 364 x 2) kJ
∆Hs =∆Hh +∆Hs →
(-1920 kJ + (- 364x2 kJ)) + (+2493) kJ
= -155.0 kJmole-1
- MgCl2 --breaking the crystal into free ions → Mg2+(g) + 2Cl-(g) ∆Hl = +2493 kJ
- Draw an energy level diagram for the dissolving of magnesium chloride (2mks)
- Calculate the heat of solution of magnesium chloride. (2mks)
-
- A solution of aqueous sodium hydroxide is added to a gas jar of nitrogen (IV) oxide and shaken. State and explain the observation made (2marks)
- The brown fumes disappear. Nitrogen (IV) oxide is an acidic gas because it can react with an alkali Forming sodium nitrate and sodium nitrite.
- Write the chemical equation for the reaction above (1 mark)
- 2NaOH(aq) + 2NO2(g) → 2NaNO3(g) + NaNO2(aq) + H2O(l)
- A solution of aqueous sodium hydroxide is added to a gas jar of nitrogen (IV) oxide and shaken. State and explain the observation made (2marks)
Download Chemistry Paper 1 Questions and Answers - Royal Exam Series Post Mock Trial Exams 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students