QUESTIONS
- State two branches of chemistry (2MKS)
- Define each of the following terms (2MKS)
- Matter
- Conductor
- Differentiate between a pure substance and a mixture (2Mks)
- State two factors that determine the choice of the method of separation of mixtures (2mks)
- Describe how you can separate a mixture of iodine, salt and sand (3mks)
- State the use of each of the following laboratory apparatus (3MKS)
- Pipette
- Deflagrating spoon
- Thistle funnel
- James a form one student wants to measure accurate volume of a solution in the laboratory State TWO apparatus that James could use (2MKS)
-
- Name two laboratory apparatus that can be made of glass (2MKS)
- State two advantages of apparatus made of glass in chemistry experiments (2MKS)
-
- Draw and name the regions of a luminous flame (3mks)
- A wooden splint was slipped through a region of a particular flame in the laboratory and was burnt as shown in the diagram below

- Name the type of flame the splint was slipped through (1mk)
- Stating the region explain why the splint was burnt the way it is shown in the diagram (2mks)
- State one disadvantage of using the above type of flame in the laboratory (1mk)
-
- Define the term crystallization (1mk)
- State two applications of crystallization (2mks)
-
- What is solvent extraction (1mk)
- Describe how oil is extracted from ground nut seeds (3mks)
- For each of the following, state the type of change observed (4mks)
- Melting and cooling candle wax
- Heating and cooling zinc oxide
- Burning magnesium in air
- Heating hydrated copper (ii) sulphate
- Study the diagram below and answer the questions that follow (5mks)
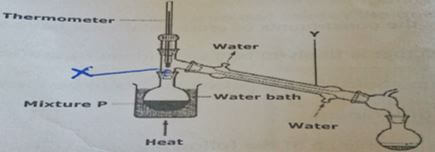
- Name each of the parts X and Y
- What is the name given to the method used above for the separation of mixture P?
- State two industrial applications of the method mentioned above
- Suppose the mixture P was paraffin and water, which apparatus could have been used to separate them?
- State the effect of impurities on (2mks)
- Melting point
- Boiling point
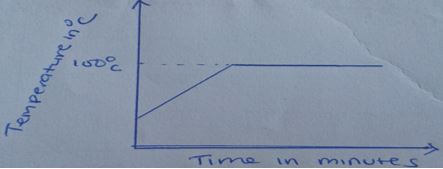
- The diagram below shows the heating curve of pure water On the same diagram, sketch the graph if water containing impurities was used (1mks)
-
- State the kinetic theory of matter (1mk)
- By use of a clear (theoretical model) diagram, explain the nature of particles in the three states of matter (3mks)
MARKING SCHEME
- State two branches of chemistry (2MKS)
- organic chemistry
- analytical chemistry
- inorganic chemistry
- biochemistry
- Define each of the following terms (2MKS)
- Matter
- anything that has mass and occupies space
- Conductor
- substance which allow electrical energy to flow through them
- Matter
- Differentiate between a pure substance and a mixture (2Mks)
- a pure substance contains only one kind of particle or fixed chemical composition while a mixture is a substance made up of two or more substances combined
- State two factors that determine the choice of the method of separation of mixtures (2mks)
- density of the particles forming the mixture
- the solubility of the particles forming the mixture
- magnetism
- boiling point
- Describe how you can separate a mixture of iodine, salt and sand (3mks)
- heat the mixture and condence the vapour produced to collect deposits of iodine
- add water to the remaining mixture then stir for salt to dissolve
- filter to obtain sand as residue then heat the filtrate to saturation to obtain salt crystals
- State the use of each of the following laboratory apparatus (3MKS)
- Pipette
- used to measure faily accurate volumes of liquids in the laboratory
- Deflagrating spoon
- used for holding substances being burned in glass jars
- Thistle funnel
- used for delivering liquid substances in reaction vessels
- Pipette
- James a form one student wants to measure accurate volume of a solution in the laboratory State TWO apparatus that James could use (2MKS)
- pipette
- burette
- syringe
- volumetric flask
-
- Name two laboratory apparatus that can be made of glass (2MKS)
- conical flask
- beaker
- State two advantages of apparatus made of glass in chemistry experiments (2MKS)
- easy to clean
- can be used when heating substances
- Name two laboratory apparatus that can be made of glass (2MKS)
-
- Draw and name the regions of a luminous flame (3mks)

- A wooden splint was slipped through a region of a particular flame in the laboratory and was burnt as shown in the diagram below

- Name the type of flame the splint was slipped through (1mk)
- non-luminous flame
- Stating the region explain why the splint was burnt the way it is shown in the diagram (2mks)
- the outter pale blue zone of th non luminous flame is the hottest region as compared to the inner regions
- State one disadvantage of using the above type of flame in the laboratory (1mk)
- it is not easily visible hence accidents are easy to make
- Name the type of flame the splint was slipped through (1mk)
- Draw and name the regions of a luminous flame (3mks)
-
- Define the term crystallization (1mk)
- the process of obtaining crytsals from a saturated solution
- State two applications of crystallization (2mks)
- extraction of salt from salty water
- extraction of medical substances from plants
- Define the term crystallization (1mk)
-
- What is solvent extraction (1mk)
- the process of removing a substance from a solution or mixture by dissolving it in another
- Describe how oil is extracted from ground nut seeds (3mks)
- crush the ground nut seeds then add propane. The dissolves in propane . remove the solid part then put the resulting solution in the sun. The propane evaporates leaving the oil.
- What is solvent extraction (1mk)
- For each of the following, state the type of change observed (4mks)
- Melting and cooling candle wax - temporary physical change
- Heating and cooling zinc oxide - temporary physical change
- Burning magnesium in air - temporary chemical change
- Heating hydrated copper (ii) sulphate - temporary chemical change
- Study the diagram below and answer the questions that follow (5mks)

- Name each of the parts X and Y
- x - fructionating column
- y - liebig condenser
- What is the name given to the method used above for the separation of mixture P?
- fractional distillation
- State two industrial applications of the method mentioned above
- in manufacture of nitrogen and oxygen
- separating the component of crude oil
- Suppose the mixture P was paraffin and water, which apparatus could have been used to separate them?
- separating funnel
- Name each of the parts X and Y
- State the effect of impurities on (2mks)
- Melting point
- lowers the melting point
- Boiling point
- raises the boiling point
- Melting point
- The diagram below shows the heating curve of pure water On the same diagram, sketch the graph if water containing impurities was used (1mks)

-
- State the kinetic theory of matter (1mk)
- matter is made up of small particles that are in continuous state of random motion
- By use of a clear (theoretical model) diagram, explain the nature of particles in the three states of matter (3mks)
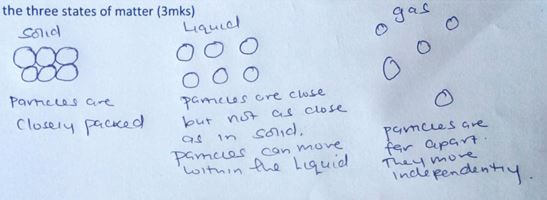
- State the kinetic theory of matter (1mk)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Questions and Answers - Form 1 Mid Term 2 Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

