
Topic Review
Matter
Matter is anything that has mass and occupies space.
States of Matter
Matter is anything that has weight/ mass and occupies space/ volume. Naturally, there are basically three states of matter.
- Solid -e.g. soil, sand, copper metal, bucket, ice.
- Liquid- e.g. water, Petrol, ethanol/alcohol, Mercury (liquid metal).
- gas- e.g. Oxygen, Nitrogen ,Water vapour.
A solid is made up of particles which are very closely packed. It thus has a definite/fixed shape and fixed/definite volume /occupies definite space. It has a very high density.
A liquid is made up of particles which have some degree of freedom. It thus has no definite/fixed shape. It takes the shape of the container it is put. A liquid has fixed/definite volume/occupies definite space.
A gas is made up of particles free from each other. It thus has no definite/fixed shape. It takes the shape of the container it is put. It has no fixed/definite volume/occupies every space in a container.
Separation of mixture
A mixture is a combination of two or more substances that can be separated by physical means. Simple methods of separating mixtures at basic chemistry level include:
- Sorting/picking- this involve physically picking one pure substance from a mixture with another/other. e. g. sorting maize from maize beans mixture.
- Decantation- this involve pouring out a liquid from a solid that has settled /sinking solid in it. e. g. Decanting water forms sand .
- Filtration -this involves sieving /passing particles of a mixture through a filter containing small holes that allow smaller particle to pass through but do not allow bigger particle to pass through.
- Skimming- this involve scooping floating particles. E.g. cream from milk
Metals and non-metals
Metals are shiny, ductile(able to form wires), malleable(able to form sheet) and coil without breaking. E.g. Iron, gold, silver, copper. Mercury is the only liquid metal known. Non-metals are dull, not ductile (do not form wires), not malleable (do not form sheet) and break on
coiling/brittle. E.g. Charcoal, Sulphur, pla-stics.
Conductors and non-conductors
A conductor is a solid that allow electric current to pass through. A non-conductor is a solid that do not allow electric current to pass through.
All metals conduct electricity. All non-metals do not conduct electricity except carbon graphite .
Drugs and Drug Abuse
A drug is a substance which is used as medicine or in a medicine. Some drugs occur naturally in some plants and animals or are man-made.
Drugs are administered in specified dosages as prescribed by a medical doctor. When an overdose or underdose of a drug is administered or when a drug is used for a purpose which it is not meant for, the condition is reffered to as drug abuse. Drug abuse has harmful effects on the state of health of the user.
The following are some of the drugs that are mostly abused which are also very addictive.
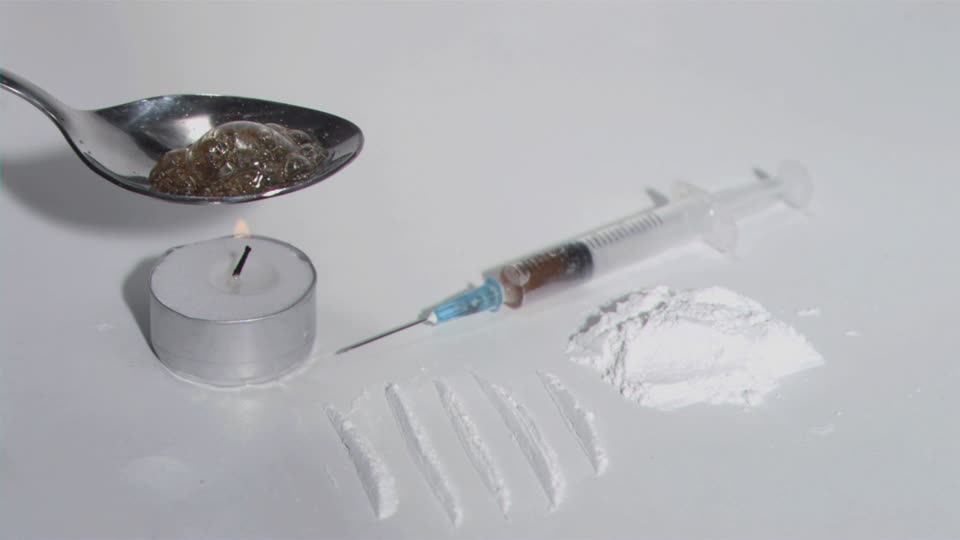
The images shows the hard drugs that are abused. These drugs are very addictive.
The image below shows cocaine in powder form

The image below shows heroin powder and how it is prepared for use.
The image below shows the Cannabis sativa plant


What is Chemistry?
Chemistry is a branch of science in which the composition and properties of matter are studied.
The Role of Chemistry in the Society
- Chemistry is used in the following:
- Washing/cleaning with soap:
Washing/cleaning is a chemical process that involves interaction of water, soap and dirt so as to remove the dirt from a garment. - Understanding chemicals of life
Living thing grow, respire and feed. The formation and growth of cells involve chemical processes in living things using carbohydrates, proteins and vitamins. - Baking:
Adding baking powder to dough and then heating in an oven involves interactions that require understanding of chemistry. - Medicine:
Discovery, test, prescription and dosage of drugs to be used for medicinal purposes require advanced understanding of chemistry. - Fractional distillation of crude oil:
Crude oil is fractional distilled to useful portions like petrol, diesel, kerosene by applying chemistry. - Manufacture of synthetic compounds/substances
Large amounts of plastics, glass, fertilizers, insecticides, soaps, cements, are manufactured worldwide. Advanced understanding of the chemical processes involved is a requirement. - Diagnosis/test for abnormal body functions.
If the body is not functioning normally, it is said to be sick/ill. Laboratory test are done to diagnose the illness/sickness.
- Washing/cleaning with soap:
- The following career fields require Chemistry as one of subject areas of advanced/specialized study:
- Chemical engineering/chemical engineer
- Veterinary medicine/Veterinary doctor
- Medicine/Medical doctor/pharmacist/nurse
- Beauty/Beautician
- Teaching/Chemistry teacher.


Chemistry Laboratory
Apparatus
Apparatus for measuring volume
- Measuring cylinder
Measuring cylinders are apparatus used to measure volume of liquid/ solutions.
They are calibrated/ graduated to measure any volume required to the maximum.
Measuring cylinders are named according to the maximum calibrated/graduated volume e.g.
“10ml” measuring cylinder is can hold maximum calibrated/graduated volume of “10mililitres” /“10 cubic centimetres”
“50ml” measuring cylinder is can hold maximum calibrated/graduated volume of “50mililitres” /“50 cubic centimetres”
“250ml” measuring cylinder is can hold maximum calibrated/graduated volume of “250mililitres” /“250 cubic centimetres”
“1000ml” measuring cylinder is can hold maximum calibrated/graduated volume of “1000mililitres”/“1000 cubic centimetres”
- Burette
Burette is a long and narrow/thin apparatus used to measure small accurate and exact volumes of a liquid solution. It must be clamped first on a stand before being used. It has a tap to run out the required amount out. They are calibrated/ graduated to run out small volume required to the maximum 50ml/50cm3.
The maximum 50ml/50cm3 calibration/ graduation reading is at the bottom. This ensure the amount run out from a tap below can be determined directly from burette reading before and after during volumetric analysis. Burettes are expensive and care should be taken when using them.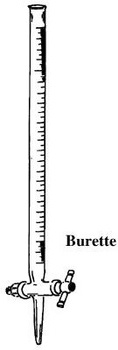
- Pipette - Pipette is a long and narrow/thin apparatus that widens at the middle used to measure and transfer small very accurate/exact volumes of a liquid solution. It is open on either ends.
The maximum 25ml/25cm3 calibration/ graduation mark is a visible ring on one thin end. To fill a pipette to this mark, the user must suck up a liquid solution upto a level above the mark then adjust to the mark using a finger. This requires practice. - Pipette filler - Pipette filler is used to suck in a liquid solution into a pipette instead of using the mouth. It has a suck, adjust and eject button for ensuring the exact volume is attained. This requires practice.
- Pipette - Pipette is a long and narrow/thin apparatus that widens at the middle used to measure and transfer small very accurate/exact volumes of a liquid solution. It is open on either ends.
- Volumetric flask.
A volumetric flask is thin /narrow but widens at the base/bottom. It is used to measure very accurate/exact volumes of a liquid solution.
The maximum calibration / graduation mark is a visible ring.
Volumetric flasks are named according to the maximum calibrated/graduated volume e.g.
“250ml” volumetric flask has a calibrated/graduated mark at exact volume of “250mililitres” /“250centimetres”
“1l” volumetric flask has a calibrated/graduated mark at exact volume of “one litre” /“1000 cubic centimeters”
“2l” volumetric flask has a calibrated/graduated mark at exact volume of “two litres” /“2000 cubic centimeters”
- Dropper/teat pipette
A dropper/teat pipette is a long thin/narrow glass/rubber apparatus that has a flexible rubber head. A dropper/teat pipette is used to measure very small amount/ drops of liquid solution by pressing the flexible rubber head. The numbers of drops needed are counted by pressing the rubber gently at a time.
Apparatus for measuring mass
- Beam balance
A beam balance has a pan where a substance of unknown mass is placed. The scales on the opposite end are adjusted to “balance” with the mass of the unknown substance. The mass from a beam balance is in grams.
- Electronic/electric balance.
An electronic/electric balance has a pan where a substance of unknown mass is placed. The mass of the unknown substance in grams is available immediately on the screen.
Apparatus for measuring temperature
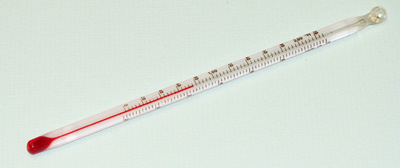
A thermometer has alcohol or mercury trapped in a bulb with a thin enclosed outlet for the alcohol/mercury in the bulb. If temperature rises in the bulb, the alcohol /mercury expand along the thin narrow enclosed outlet. The higher the temperature, the more the expansion.
Outside, a calibration /graduation correspond to this expansion and thus changes in temperature.
A thermometer therefore determines the temperature when the bulb is fully dipped in to the substance being tested. To determine the temperature of solid is thus very difficult.
Apparatus for measuring time
The stop watch/clock is the standard apparatus for measuring time. Time is measured using hours, minutes and second. Common school stop watch/clock has start, stop and reset button for determining time for a chemical reaction. This requires practice.

Apparatus for scooping
- Spatula
A spatula is used to scoop solids which do not require accurate measurement. Both ends of the spatula can be used at a time. A solid scooped to the brim is “one spatula end full” A solid scooped to half brim is “half spatula end full”.
- Deflagrating spoon
A deflagrating spoon is used to scoop solids which do not require accurate measurement mainly for heating. Unlike a spatula, a deflagrating spoon is longer.
Apparatus for putting liquids/solid for heating.
- Test tube.
A test tube is a narrow/thin glass apparatus open on one side. The end of the opening is commonly called the “the mouth of the test tube”.
- Boiling/ignition tube.
A boiling/ignition tube is a wide glass apparatus than a test tube open on one side. The end of the opening is commonly called the “the mouth of the boiling/ignition tube”.
- Beaker.
Beaker is a wide calibrated/graduated lipped glass/plastic apparatus used for transferring liquid solution which do not normally require very accurate measurements Beakers are named according to the maximum calibrated/graduated volume they can hold e.g.
“250ml” beaker has a maximum calibrated/graduated volume of “250mililitres” /“250 cubic centimeters”
“1l” beaker has a maximum calibrated/graduated volume of “one litre” /“1000 cubic centimeters”
“5 l” beaker has a maximum calibrated/graduated volume of “two litres” /“2000 cubic centimeters”
- Conical flask.
A conical flask is a moderately narrow glass apparatus with a wide base and no calibration/graduation. Conical flasks thus carry/hold exact volumes of liquids that have been measured using other apparatus. It can also be put some solids. The narrow mouth ensures no spillage.
Conical flasks are named according to the maximum volume they can hold e.g. “250ml” Conical flasks hold a maximum volume of “250mililitres” /“250 cubic centimeters”
“500ml” Conical flasks hold a maximum volume of “500ml” /“1000 cubic centimeters”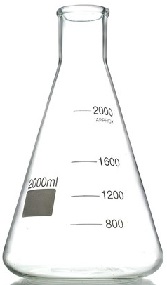
- Round bottomed flask
A round bottomed flask is a moderately narrow glass apparatus with a wide round base and no calibration/graduation. Round bottomed flask thus carry/hold exact volumes of liquids that have been measured using other apparatus. The narrow/thin mouth prevents spillage. The flask can also hold (weighed) solids. A round bottomed flask must be held/ clamped when in use because of its wide narrow base.
- Flat bottomed flask
A flat bottomed flask is a moderately narrow glass apparatus with a wide round base with a small flat bottom. It has no calibration/graduation. Flat bottomed flasks thus carry/hold exact volumes of liquids that have been measured using other apparatus. The narrow/thin mouth prevents spirage. They can also hold (weighed) solids. A flat bottomed flask must be held/clamped when in use because it’s flat narrow base is not stable.
Apparatus for holding unstable apparatus (during heating).
- Tripod stand
A tripod stand is a three legged metallic apparatus which unstable apparatus are placed on (during heating).Beakers. Conical flasks, round bottomed flask and flat bottomed flasks are placed on top of tripod stand (during heating).
- Wire gauze/mesh
Wire gauze/mesh is a metallic/iron plate of wires crossings. It is placed on top of a tripod stand:- Ensure even distribution of heat to prevent cracking glass apparatus
- Hold smaller apparatus that cannot reach the edges of tripod stand

- Clamp stand
A clamp stand is a metallic apparatus which tightly hold apparatus at their “neck” firmly. A clamp stand has a wide metallic base that ensures maximum stability. The height and position of clamping is variable. This require practice
- Test tube holder
A test tube holder is a hand held metallic apparatus which tightly hold test/boiling/ignition tube at their “neck” firmly on the other end. Some test tube holders have wooden handle that prevent heat conduction to the hand during heating.
- Pair of tong.
A pair of tong is a scissor-like hand held metallic apparatus which tightly hold firmly a small solid sample on the other end.
- Gas jar
A gas jar is a long wide glass apparatus with a wide base. It is open on one end. It is used to collect/put gases. This requires practice.
Apparatus for holding/directing liquid solutions/funnels (to avoid spillage).
- Filter funnel
A filter funnel is a wide mouthed (mainly plastic) apparatus that narrow drastically at the bottom to a long extension. When the long extension is placed on top of another apparatus, a liquid solution can safely be directed through the wide mouth of the filter funnel into the apparatus without spirage. Filter funnel is also used to place a filter paper during filtration.
- Thistle funnel
A thistle funnel is a wide mouthed glass apparatus that narrow drastically at the bottom to a very long extension. The long extension is usually drilled through a stopper/cork. A liquid solution can thus be directed into a stoppered container without spillage.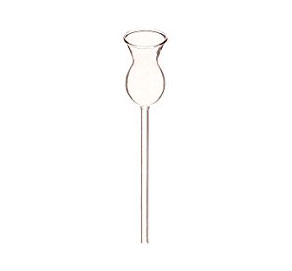
- Dropping funnel
A dropping funnel is a wide mouthed glass apparatus with a tap that narrow drastically at the bottom to a very long extension.
The long extension is usually drilled through a stopper/cork. A liquid solution can thus be directed into a stoppered container without spillage at the rate determined by adjusting the tap.
- Separating funnel
A separating funnel is a wide mouthed glass apparatus with a tap at the bottom narrow extension. A liquid solution can thus be directed into a separating funnel without spillage. It can also safely be removed from the funnel by opening the tap. It is used to separate two or more liquid solution mixtures that form layers/immiscible. This requires practice.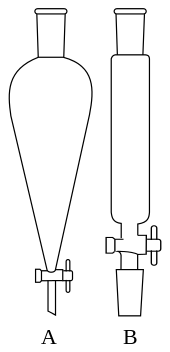
The Bunsen Burner
The bunsen burner consists of three major parts. These are the chimney or barrel or tube, the collar and the base.
The image below shows parts of a bunsen burner
Notice the base, collar, chimney/burner tube/barrel
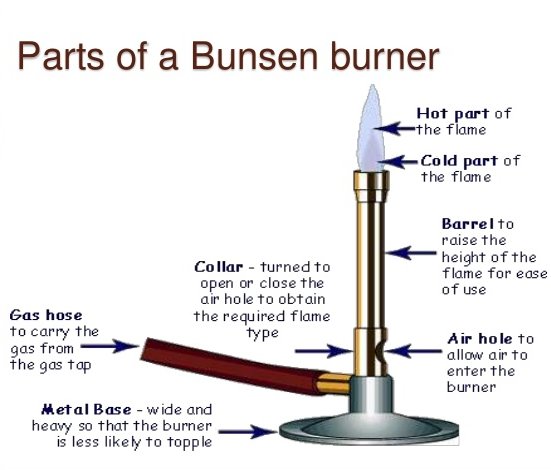
The chimney is a hollow metallic cylinder with an air hole near its lower end. The collar is a metal ring with an air hole whose diameter is the same size as that of the hole in the chimney. The diameter of the collar is slightly bigger than that of the chimney so that the chimney can just fit into it. The base is made up of thick metallic material into which a small hollow metal with a jet is fitted. The bunsen burner is normally connected to an external source of laboratory gas by rubber tubing.
Funtions of the Different Parts of the Bunsen Burner
The rubber tubing connects the inlet to an external source of laboratory gas. The jet allows the laboratory gas into the chimney. The collar is used to regulate the amount of air entering the chimney. The air hole in the chimney allows air to enter and mix with the laboratory gas from the jet. This mixture of gases (laboratory gas and air), when ignited burn at the end of the chimney to produce a flame. The flame is used to provide heat.
When in use, a Bunsen burner produces two types of flames depending on the amount of air allowed into the chimney.
The image below shows the two types of flame
Image (a)is non-luminous while (b) is luminous
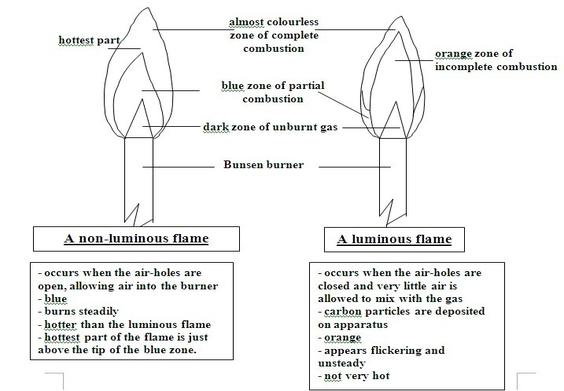
- When the air hole is closed, no air enters the chimney.
- The flame produced is bright yellow and large.
- It gives out much light and is described as a luminous flame
- The colour of the flame is not uniform and four zones are seen.
- The blue region occurs at the bottom of the flame. Here, air near the flame rises rapidly and mixes with the burning gas.
- This makes burning more complete than in the other parts of the flame.
- The almost colourless region of the flame consists mainly of unburnt gases.
- The luminous bright yellow region consists of unburnt tiny particles of white-hot solid carbon which give out light.
- The unburnt carbon particles produce the black soot which makes apparatus dirty during heating.
- Air supply in this region is limited and hence there is incomplete combustion of the gas.
- In the thin outer region, the gas burns completely because it mixes with plenty of air. This region is however difficult to see.
- When the air hole is slowly opened, more air enters the chimney.
- The bright yellow colour of the luminous flame gradually disappears. When the air hole is fully opened, a lot of air enters the chimney and mixes with the laboratory gas.
- The mixture of gases burn more quickly and completely. The flame obtained is blue in colour and is described as non-luminous flame.
- The non-luminous flame does not give out much light.
- The non-luminous flame has three regions as follows.
- The almost colourless region consists of unburnt gases.
- The greenish-blue region in the middle of the flame contains partially burnt gases due to insufficient supply of air.
- In the outer pale blue region, the gases burn completely because there is plenty of air.
- The non-luminous flame gives out only a little light because it does not contain white-hot carbon particles.
Characteristic differences between luminous and non-luminous flame
| Luminous flame | Non-luminous flame |
|
1. Produced when the air holes are fully/completely closed. |
1. Produced when the air holes are fully/completely open. |
|
2. when the air holes are fully/ completely closed there is incomplete burning/ combustion of the laboratory gas |
2. when the air holes are fully/ completely open there is complete burning/ combustion of the laboratory gas |
|
3. Incomplete burning/ combustion of the laboratory gas produces fine unburnt carbon particles which make the flame sooty/smoky |
3. Complete burning/ combustion of the laboratory gas does not produce carbon particles. This makes the flame non- sooty /non- smoky. |
|
4. Some carbon particles become white hot and emit light. This flame is thus bright yellow in colour producing light. This makes luminous flame useful for lighting |
4. Is mainly blue in colour and is hotter than luminous flame. This makes non-luminous flame useful for heating |
|
5. Is larger, quiet and wavy /easily swayed by wind |
5. Is smaller, noisy and steady |
|
Luminous flame has three main regions:
|
Non-luminous flame has four main regions:
|
Safety in the Laboratory
The laboratory should always be clean, neat and with ample lighting.
When carrying out an experiment in the laboratory, it should be considered as potentially dangerous. All chemicals and apparatus should be handled with care. For example, if a piece of glass apparatus is handled carelessly, it may break and harm the user.
Since learning Chemistry emphasizes on practical work, it is necessary that certain rules are followed to ensure safety in the chemistry laboratory. The common causes of accidents in the laboratory are carelessness and ignorance. Accidents are minimised when the laboratory safety rules are observed.
Laboratory Safety Rules
- NEVER run while in the laboratory because you may harm or injure yourself and other lab users such as your fellow students.
- NEVER taste anything in the laboratory to avoid poisoning.
- Always consult your teacher before trying any experiment to avoid accidents.
- Label all the chemicals you are using to avoid confusion.
- Always use a clean spatula for scooping a substance from a container to minimise contamination.
- Always hold test-tubes or boiling tubes using a test-tube holder when heating to avoid being burnt
- When heating a substance, NEVER let the open end of the tube face yourself or anybody else because the liquid may spurt out and cause injury.
- NEVER look directly into flasks and test-tubes where reactions are taking place, because the chemicals may spurt into your eyes and cause injury.
- NEVER smell gases directly. Instead waft the gaseous fumes near your nose with your hand.
- Experiments in which poisonous gases and vapours are produced must be carried out in a fume cupboard or an open space outdoors.
- Always keep flammable substances away from flames because they easily catch fire.
- Always report accidents to teachers or the laboratory technicians immediately for necessary action to be taken.
- In case of an accident do not scramble for the same exit, because it may hinder easy escape.
- Always put off flames that are not in use in order to avoid accidents and minimise fuel wastage.
- If a chemical gets on your skin, rinse it immediately with alot of water.
- Always dispose off the chemicals already used safely to avoid explosions and contamination
- Always work on a clean bench. After completing your experiment, clean all the pieces of apparatus you have used and return them to their correct storage places.
Download INTRODUCTION TO CHEMISTRY - Chemistry Notes Form 1.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students


