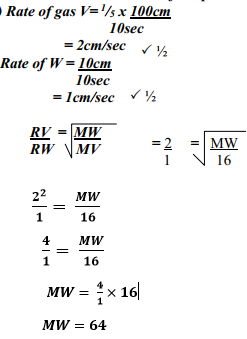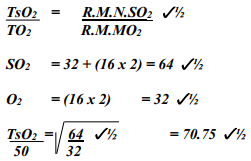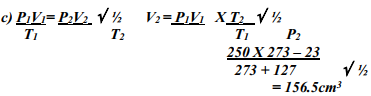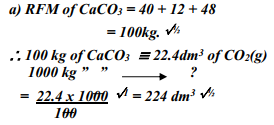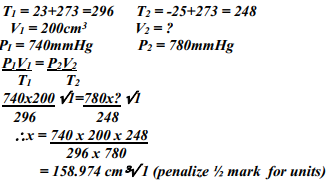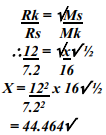Questions
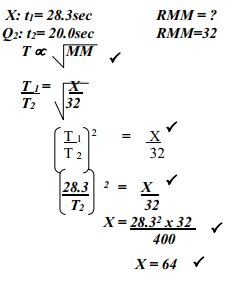
- A sample of unknown compound gas X is shown by analysis to contain Sulphur and Oxygen. The gas requires 28.3 seconds to diffuse through a small aperture into a vacuum. An identical number of oxygen molecules pass through the same aperture in 20 seconds. Determine the molecular mass of gas X (O= 16, S= 32)
-
- State Graham’s Law of diffusion
- Gas V takes 10 seconds to diffuse through a distance of one fifth of a meter. Another gas W takes the same time to diffuse through a distance of 10 cm. if the relative molecular mass of gas V is 16.0; calculate the molecular mass of W
-
- State Charles’ Law
- The volume of a sample of nitrogen gas at a temperature of 291K and 1.0 x 105 Pascals was 3.5 x 10-2 m3. Calculate the temperature at which the volume of the gas would be 2.8 x 10-2 m3 at 1.0 x 105 pascals.
- 60 cm3 of oxygen gas diffused through a porous partition in 50 seconds. How long would it take 60 cm3 of sulphur (IV) oxide gas to diffuse through the same partition under the sane conditions? (S = 32.0, O = 16.0)
-
- State Graham’s law of diffusion
- 30cm3 of hydrogen chloride gas diffuses through a porous pot in 20 seconds. How long would it take 42cm3 of sulphur(IV) oxide gas to diffuse through the same pot under the same conditions (H =1 Cl = 35.5 S = 32 O =16)
-
- State Boyles law
- Sketch a graph that represents Charles’ law
- A gas occupied a volume of 250 cm3 at -23º C and 1 atmosphere. Determine its volume at 127º C when pressure is kept constant.
- A factory produces Calcium Oxide from Calcium Carbonate as shown in the equation below:-
heat
CaCO3 (s) → CaO (s) + CO2 (g)
What volume of Carbon (IV) Oxide would be produced from 1000kg of Calcium Carbonate at s.t.p (Ca = 40, C = 12, O = 16, Molar gas volume at s.t.p = 22.4dm3) - A fixed mass of gas occupies 200 cm3 at a temperature of 23oC and pressure of 740mmHg. Calculate the volume of the gas at -25oC and 780mmHg pressure
- Gas K diffuses through a porous material at a rate of 12cm3 s-1 where as S diffuses through the same material at a rate of 7.5cm3s-1. Given that the molar mass of K is 16, calculate the molar mass of S
- State Gay Lussac’s law
-
- What is the relationship between the rate of diffusion of a gas and its molecular mass?
- A sample of Carbon (IV) Oxide takes 200 seconds to diffuse across a porous plug.
How long will it take the same amount of Carbon (II) Oxide to diffuse through the same plug?(C=12, O=16)
- Below are structures of particles. Use it to answer questions that follow. In each case only electrons in the outermost energy level are shown
key
P = Proton
N = Neutron
X = Electron- Identify the particle which is an anion
- Choose a pair of isotopes. Give a reason
- The figure below shows two gases P and Q diffusing from two opposite ends 18 seconds after the experiment
- Which of the gases has a lighter density?
- Given that the molecular mass of gas Q is 17, calculate the molecular mass of P
- Identify the particles that facilitate the electric conductivity of the following substances
- Sodium metal
- Sodium Chloride solution
- Molten Lead Bromide
- Gas B takes 110 seconds to diffuse through a porous pot, how long will it take for the same amount of ammonia to diffuse under the same conditions of temperature and pressure? (RMM of B = 34 RMM of ammonia = 17)
- A gas occupies 5dm3 at a temperature of -27oC and 1 atmosphere pressure. Calculate the volume occupied by the gas at a pressure of 2 atmospheres and a temperature of 127oC
- A fixed mass of gas occupies 200 cm3 at a temperature of 230c and a pressure of 740 mm Hg. Calculate the volume of the gas at -250c and 790 mm Hg pressure.
-
- State the Graham’s law
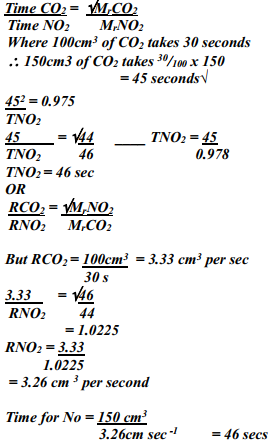
- 100cm3 of Carbon (IV) oxide gas diffused through a porous partition in 30seconds. How long would it take 150cm3 of Nitrogen (IV) oxide to diffuse through the same partition under the same conditions? (C = 12.0, N = 14.0, O = 16.0)
Answers
-
-
- The rate of diffusion of a gas is inversely proportional to the square root of its density under the same conditions of temperature and pressure
-
- The rate of diffusion of a gas is inversely proportional to the square root of its density under the same conditions of temperature and pressure
-
- The volume of a fixed mass of a gas is directly proportional to its absolute temperature at constant Pressure
-
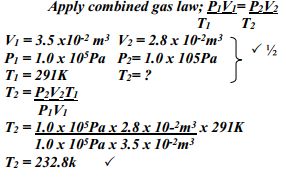
-
-
- The rate of diffusion of a fixed mass of a gas is inversely proportional to the square root of it density at constant temperature and pressure
-
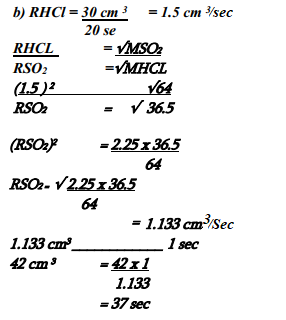
- The rate of diffusion of a fixed mass of a gas is inversely proportional to the square root of it density at constant temperature and pressure
-
- Boyles’ law For a fixed mass of a gas, volume is inversely promotional to pressure at constant temperature
-
-
-
-
- When gases combine they do so in volume which bear a simple ratio to one another and to the product if gaseous under standard temperature and pressure
-
- Rate of diffusion is whereby proportional to molecular mass of a gas. √1
-
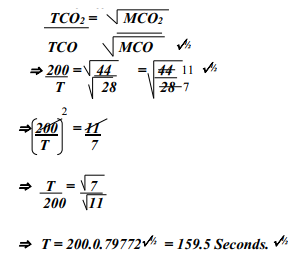
-
- Y √1
- Z and W √1 have same atomic number but different mass number. √1
-
- Gas P
-
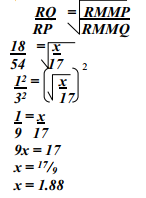
-
- Delocalized electrons
- Mobile ions
- Mobile ions
-
-
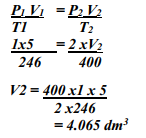
-
-
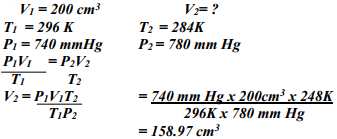
- 601 √1
-
-
- Grahams law states
Under the same conditions of pressure and temperature, the rate of diffusion of a gas is inversely proportional to the square root of its density -
- Grahams law states
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Gas Laws Questions and Answers - Chemistry Form 3 Topical Revision.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students