Questions
- The apparatus below was set-up to show the catalytic oxidation of ammonia. Study the diagram and answer the questions that follow:-
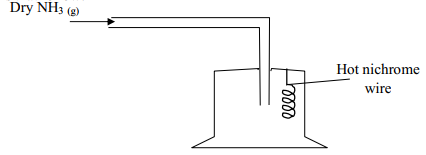
- Write an equation for the reaction that takes place
- Why is it necessary to have a hot nichrome wire in the gas jar
- Write the formula of the complex ion formed when excess ammonia gas is passed through a solution containing Zn2+ ions
- The diagram below shows the catalytic oxidation of ammonia gas. Use it to answer the questions that follow:-
- What metal could rod M be made of?
- State and explain two observations made inside the conical flas
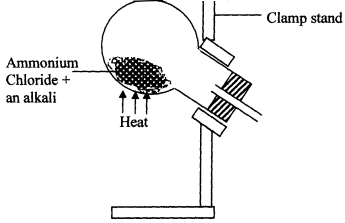
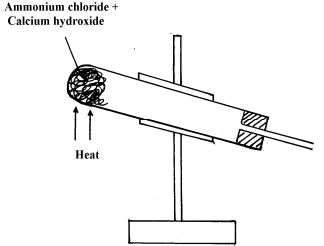
- Ammonia gas is prepared in the laboratory by the action of an alkali on an ammonium salt.
A student wanted to prepare a sample of ammonia gas in the laboratory.- Give one alkali that can be used in the above experiment
- Write an equation for the reaction that takes place in the above experiment
-
- Explain the importance of the high percentage of nitrogen in air
- Why is nitrogen used for storage of semen in artificial insemination?
- The diagram below is used in preparation of a gas in the laboratory. Answer the questions that follow;
- Name gas X …………………………………………………………………………..
- State one physical property which makes it possible for the gas to be collected as shown*
- State one commercial use of gas X
- Study the flow charts below and use them to answer the questions that follow:
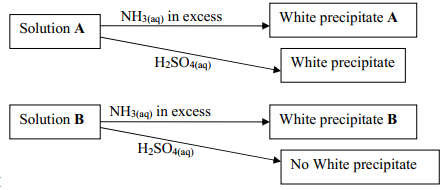
- Identify possible cations present in:
- Solution A
- Solution B
- State and explain the observations made when a sample of dry white precipitate B is heated in a test-tube
- Identify possible cations present in:
- The set-up below is an arrangement showing how metals react with nitrogen (IV) oxide.
Study it and answer the questions that follow:-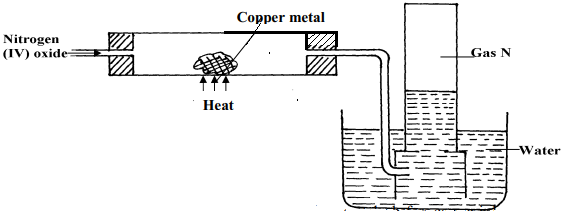
- Nitrogen (IV) oxide is passed through the combustion tube before copper is heated. Give a reason for this
- State the observations that would be made at the end of the experiment in the combustion tube
- Name gas N ……………………………………………………………………..
-
- In haber process hydrogen and nitrogen react in the presence of finely divided iron catalyst.
Explain why the catalyst is finely divided - A mixture of N2, H2 and NH3 was bubbled through 0.2M hydrochloric acid solution.
The final concentration of the acid was found to be 0.1M. Give explanation
- In haber process hydrogen and nitrogen react in the presence of finely divided iron catalyst.
- In an experiment, a few drops of concentrated nitric acid were added to aqueous iron (II) sulphate in a test-tube. Excess ammonia solution was then added to the mixture
- State the observations that were made when:-
- Concentrated nitric acid was added to aqueous iron (II) sulphate
- Excess ammonia was added to the mixture
- Write an ionic equation for the reaction which occurred in a (ii) above
- State the observations that were made when:-
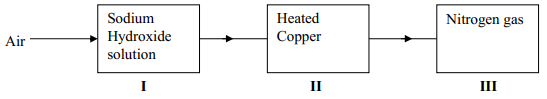
- The chart below shows a summary for the preparation of nitrogen gas from air
- What is the purpose of the sodium hydroxide?
- Write an equation for the reaction taking place in chamber II
- The nitrogen gas obtained is not pure. Explain
- Dilute nitric acid is added to excess green solid. Effervescence occurs and a blue solution is formed.
When excess ammonia solution is added to a sample of the solution a deep blue solution is formed- Identify the anion and cation in the green solid:
- Write an ionic equation for the reaction forming deep blue solution
-
- The diagram below is a set-up for preparation and collection of a gas. Study it answer the questions that follow:
- Identify gas X ………………………………………………………….
- Write an equation for the formation of gas X
- What precaution should be observed when preparing gas X by the above method?
- Describe the suitable drying agent for gas X
- How can one confirm that the gas collected is gas X?
- State two physical properties of gas X
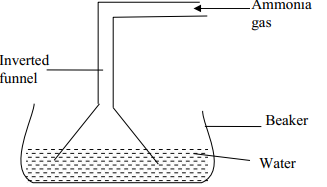
- The diagram below is a set-up used in preparation of ammonia solution. Study it and answer the questions that follow
- What is the purpose of the filter funnel in the set-up above?
- What would happen if a delivery tube was used in place of the filter funnel?
- What observation would be made on litmus paper placed into the solution in the beaker at the end of the experiment?
- The diagram below is a set-up for preparation and collection of a gas. Study it answer the questions that follow:
- The following flow chart shows the industrial manufacture of Nitric (V) acid.
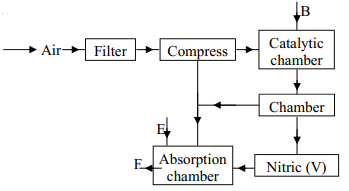
- Identify substance B, C, E and F.
- Describe what happens in the catalytic chamber.
- State what takes place in chamber D.
- 60 – 65% nitric (V) acid is produced in the absorption chamber. Describe how the acid can be concentrated.
- State why nitric (V) acid is stored in dark bottles.
- Copper reacts with nitric (V) acid and not hydrochloric acid. Explain.
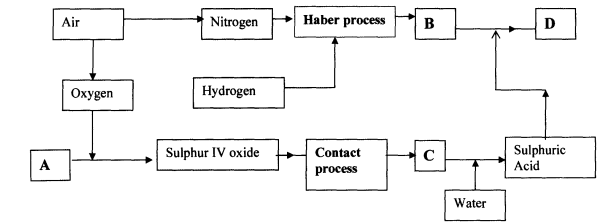
- The flow chart below illustrates two industrial processes, Haber process and the Contact process:
-
- Give the name of the process by which air is seperated into oxygen and nitrogen
- Apart from oxygen and nitrogen gases produced from process (a)(i) Name one other gas produced
- Name the substances represented by the letters A, B, C and E
- Name the catalysts used in:
- Haber Process ……………………………………………………………………..
- Contact Process ……………………………………………………………………..
- Explain the role of the catalysts in both the Haber and the Contact processes
- Write a chemical equation for the formation of compound B
- Calculate the percentage by mass of the nitrogen present in compound D
- Give one major use of compound E
-
-
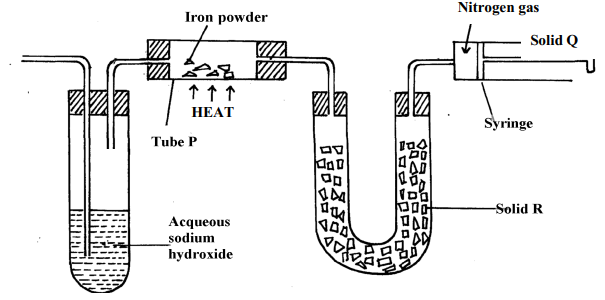
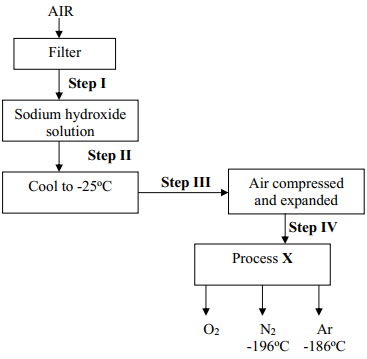
- The diagram below represents a set-up used to obtain nitrogen from air. Study it and answer the questions that follow:-
- Name solid Q ............................................................................................................
- What is the purpose of sodium hydroxide
- Write an equation for the reaction which took place in tube “P”
- Give the name of one impurity in the nitrogen gas obtained
- Give a reason why liquid nitrogen is used for storage of semen for artificial insemination
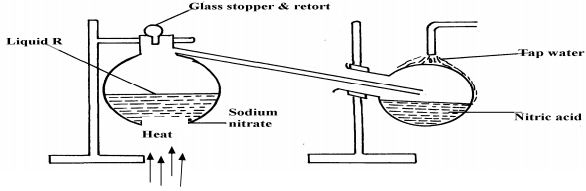
- The set-up below was used to prepare nitric acid.
- Give the name of liquid ‘R’ ....................................................................................
- Write an equation for the reaction taking place in the flask.
- Explain the following:-
- Nitric acid is stored in dark bottles
- The reaction between copper metal with 50% nitric acid in an open tube gives brown fumes
- The diagram below represents a set-up used to obtain nitrogen from air. Study it and answer the questions that follow:-
- Study the flow chart below and answer the questions which follow:
- Give one source of the following raw materials (s)
- Nitrogen gas ………………………………………………………………………………..
- Hydrogen gas …………………………………………………………………………………..
- State three conditions required in process I
- Name: catalyst P…………………………………………………………………
Gas M……………………………………………………………………..…… - Write chemical equations for;
- Formation of gas M
- The reaction in the absorption tower
- Give two reasons why step IV is necessary
- Describe how you would test if a given liquid is a nitrate
- Give three uses of nitric acid
- Give one source of the following raw materials (s)
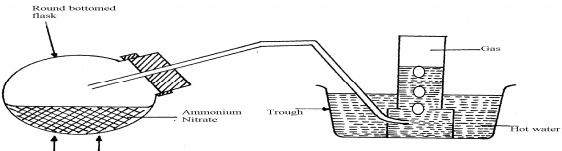
- The diagram below shows the apparatus for the laboratory preparation of one of the oxides of Nitrogen
-
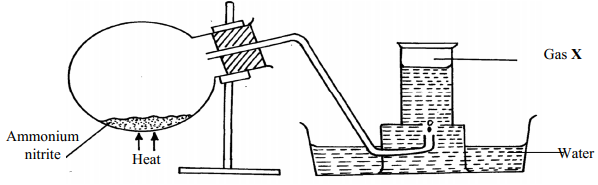
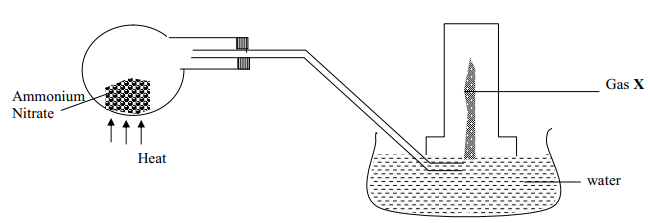
- Name the gas being produced
- Write the equation for the thermal decomposition of ammonium Nitrate
- The gas is being collected over hot water. Explain
- State and explain the observations made when burning sulphur is lowered into a gas jar containing the gas
-
- Name the catalyst used during catalytic oxidation of ammonia
- Nitrogen (IV) oxide is the final product during catalytic oxidation of ammonia.
Write a chemical equation for its formation - State two physical differences between Nitrogen (I) oxide and Nitrogen (IV) Oxide
- Nitric acid is prepared in the laboratory by action of concentrated sulphuric (VI) acid on a suitable Nitrate and distilling off the Nitric acid, in all glass apparatus.
- Why must the apparatus be made of glass?
- Hot concentrated Nitric acid reacts with sulphur in the equation below:-
S(s) + 6HNO3(aq) → H2SO3(aq) + 6NO2(g) + 2H2O(l)- Identify the species :-
Oxidised ………………………… Reduced ……………………………… - Pure nitric acid is colourless but the product during its preparation is usually pale yellow.
Explain
- Identify the species :-
-
-
- Describe the process by which oxygen can be obtained from air on large scale
- The flow chart below shows the industrial manufacture of nitric (V) acid
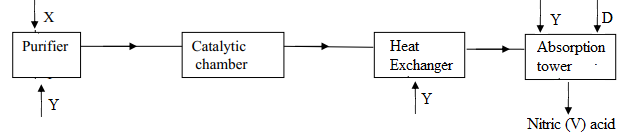
- Identify substances X and Y
- Write an equation for the reaction taking place in the absorption tower
- The concentration of the acid obtained is about 60%. How can this concentration be increased to about 65%?
- A factory uses nitric (V) acid and ammonia as the only reactants for the production of a fertilizer.
If a mass of 9600kg of fertilizer was produced, calculate the mass of ammonia gas needed
(N = 14, H = 1, O = 16)
-
- Name another substance which can be used instead of sodium hydroxide
- What is the function of filters?
- Identify the substance removed at step III
- At what temperature does liquid oxygen distil?
- Identify process X
- Describe how process X occurs
-
- State one industrial use of Nitrogen
- Air is a mixture but not a compound. Give two reasons
- Using chemical equations show the bleaching actions of chlorine and sulphur(IV)oxide
- The diagram below represents an in complete set-up for preparation of a dry sample of gas R
- Complete the set-up to show how a dry sample of gas R is collected
- Write a chemical equation for the reaction that produces gas R
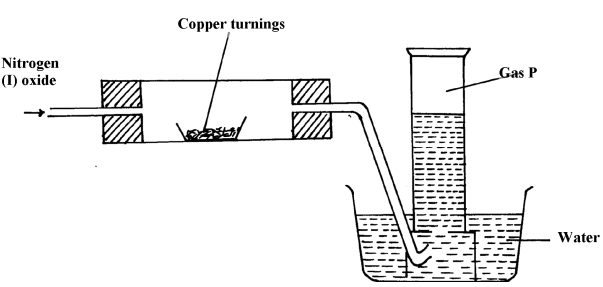
- The diagram below was used to investigate the reaction between nitrogen(I)oxide and copper turnings. Study it and answer the questions that follow:
- What has been omitted in the set-up? (show it on the diagram)
- Write a chemical equation for the reaction that took place in the combustion tube
- State one use of gas P
- When sulphur powder is heated to over 400oC the following changes are observed:- At 113oC it melts into light brown liquid. The liquid then darkens to become reddish-brown and very viscous at 160oC. Above 160oC the liquid becomes almost black. At the boiling point the liquid becomes mobile. Explain these observations
- Concentrated sodium chloride (Brine) was electrolysed using platinum electrodes. What would be the difference in terms of products at each electrode if dilute sodium chloride solution was used in place of brine. Explain
-
- Nitrogen (I) Oxide supports, combustion of burning charcoal. Write an equation to show this reaction
- Ammonium nitrate can be heated to give off nitrogen (I) Oxide. However, a mixture of NH4Cl and NaNO3 is preferred. Explain
- Ammonia turns wet red litmus paper blue. Which ion is responsible for this reaction
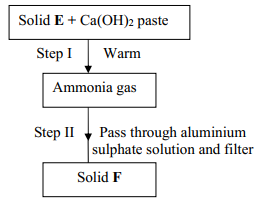
- Study the scheme below and answer the questions that follow:
- Name solids E and F
- Write down a balanced equation for the reactions that lead to formation of solid F
- When a few drops of aqueous ammonia were added to a colourless solution X, a white precipitate was formed. On addition of more aqueous ammonia, the white precipitate dissolved to a colourless solution Q
- Name the white precipitate formed
- Write formula of the complex ion present in the colourless solution Q
- Write an ionic equation for the formation of the white precipitate
- The first step in the industrial manufacture of nitric cid is the catalytic oxidation of ammonia gas.
- What is the name of the catalyst used?
- Write the equation for the catalytic oxidation of ammonia gas.
- Nitric acid is used to make ammonium nitrate. State one use of ammonium nitrate.
- Explain what is observed when ammonia gas is bubbled into Copper (II) sulphate solution till in excess.
-
- State the conditions under which nitrogen react with hydrogen to form ammonia during Haber process
- When dry ammonia gas is passed over hot copper (II) Oxide, a shinny brown residue and a colourless droplets are formed. Explain these two observations
- Study the flow chart below and answer the questions that follow
- State the observation made when ammonia is passed over heated Copper (II) Oxide
- Identify:-
- Gas A ………………………………………….………
- Liquid B ………………………………..…………………
Answers
-
- 4HN3 (g) + 5O2 (g) → 4NO(g) + 6H2O(g)
- Act as catalyst
- Zn(NH3)42+
-
- Platinum/ copper
- - Brown fumes √
- Hot rod m continues to glow red
- NO formed reacts with oxygen to form NO2 (brown flames)
- Reaction highly exothermic
-
- Calcium hydroxide
- Ca(OH)2(g) + 2NH4Cl(g) → 2NH3(g) + CaCl2 + 2H2O(l)
-
- It neutralizes air to prevent violent combustion reaction from occurring.
- Its inert and have very low b.pt of -196oC
-
- X is Nitrogen. √1
- It is less dense than air. √½
- – In preservation of semen in artificial insemination. √1
-
-
- Solution A contains Pb2+(aq) ions √½
- Solution B contains Al3+(aq) ions. √½
- – A colourless liquid at cooler parts √1 of test-tube is formed.
- A white reside remains in the test-tube. √1
-
-
- to expel air that is in the combustion tube so that oxygen in it does not react with hot copper√1
- brown√ ½ copper metal will change to black√ ½
- nitrogen √1
-
- To increase the surface area over which the reaction occurs hence increased rate of reaction.
- NH3 is basic and reacts with some moles of the acid hence reduction in concentration
-
-
- The solution changes from green √1 to brown √1 (1 mk)
- A brown √1 precipitate is formed. (1 mk) 3
- Fe3+(aq) + 3OH-(aq) → Fe(OH)3(s) √1 (1 mk)
-
-
- – Absorbs carbon (IV) oxide from√1 the air. (1 mk)
- 2 Cu(s) + O2(g) → 2CuO(s) √1 (1 mk) 3
- Because it has the rare gases. √1 (1 mk)
-
- Anion – CO32-
Cation – Cu2+ - Cu2+ + 4NH3 → {CuNH3)4}2+
- Anion – CO32-
-
-
- Nitrogen (I) Oxide
- NH4NO3 (s) → N2O(g) + 2H2O(g)
- NH4NO3 should not be heated further if the quantity remaining is small because it may explode or A mixture of NH4Cl & KNO3 can be used instead of NH4NO3 leading to double decomposition taking place safely without explosion
- An hydrous calcium chloride in a u-tube
- Reacts with oxygen to form brown fumes of Nitrogen (IV) Oxide
2N2O(g) + O2(g) → 2NO2(g) - – Has no colour
- Has a slight sweet smell
- Fairly soluble in water
- Denser than air
-
- Provides a large surface area for the absorption of ammonia gas by the water or prevent “bricking” back of water
- Water would brick back into the hot preparation flask causing it to crack or break/an explosion can occur
- Red litmus paper would turn to blue, blue litmus paper remains blue each
-
-
- B – ammonia gas ✓1
C - nitrogen (II) oxide (NO) ✓1
E – water ✓1
F – unreacted gases ✓1 - The mixture of ammonia and air is passed through heated/ catalyst where ammonia (II) is oxidized to nitrogen (II) oxide. ✓1
- Gases are cooled and air passed through heated/ catalyst where ammonia is further oxidized to nitrogen(IV) oxide. ✓1
- Fractional distillation, ✓½
Water with a lower boiling point ✓½ than nitric (V) acid, distills left leaving the concentrates acid. - To prevent sunlight from entering the bottle and decomposing the acid.
- Concentrated nitric acid is a strong oxidizing agent which is capable of oxidizing copper to soluble copper(II) ions, whereas hydrochloric acid is not a strong oxidizing agent and the copper does not react with the hydrochloric acid.
- B – ammonia gas ✓1
-
-
- Fractional distillation
- Argon
- A - Sulphur
B - Ammonia gas
C - Oteum
D - Amonium sulphate -
- Finely divided iron
- Vanadium (v) Oxide
- Speeds up the rate of reaction by lowering the activation energy
- 2NH3(g) + H2SO4(aq) → (NH4)2SO4(aq)
- R.M.M of (NH4) = 132
Mass of N = 28
% N = 28/132 x 100 = 21.212% - Used as a fertilizer
-
-
-
- Fused calcium chloride /CaO (quick lime)
- To remove carbon (IV) Oxide
- 4Fe+(s) + 3O2(g) → 3Fe2O3(s)
OR 3Fe(s) + 2O2(g) → Fe3O4(s) - Argon/Helium/Neon/Krepton
- Provide very low temperature so that the semen does not decompose /is not destroyed
-
- Concentrated sulphuric acid
- NaNO3(s) + H2SO4(l) → Na2HSO4(aq) + HNO3(aq)
OR 2NaNO3 + H2SO4(l) → Na2SO4(aq) + 2HNO3(aq)
(reject unbalanced chemical equation) -
- HNO3 is stored in brown bottles because some compounds, in the presence of sunlight, may break down or react with other compounds. Under the action of light, nitric acid slowly decomposes into oxides of nitrogen plus free oxygen, which is why they are stored in brown bottles to prevent sunlight from reaching them
- Copper reacts with 50% nitric acid to give nitrogen II Oxide which is colourless. Air oxidizes Nitrogen II oxide to Nitrogen IV oxide which is brown.
2NO(g) + O2(g) → 2NO2(g)
colourless Brown
-
-
-
- Nitrogen – Fractional distillation of liquid air –( ½ mk)
- Hydrogen – Cracking of alkanes
-Electrolysis of acidified water
- Temperature – 400oC – 500oC
Pressure – 400atm – 500atm
Catalyst – finely divided iron - Catalyst P – Nickel
Gas M – Nitrogen IV oxide -
- 2NO(g) + O2(g) → 2NO2(g)
- NO2(g) + H2O(l) → HNO2(aq) + HNO3(aq)
- To a small portion of the nitrate liquid in a test tube add equal amount o freshly prepared iron (II) sulphate followed by some drops of conc. H2SO4 slowly on the sides. If a brown ring forms on the boundary of the two solutions, a nitrate is confirmed.
- – Manufacture of nitrogenous fertilizers
- Manufacture of synthetic fibres e.g nylon
- Manufacture of explosives e.g TNT
- Manufacture of textile dyes
- Manufacture of other acids e.g. phosphoric acid
-
-
-
- Nitrogen (I) Oxides.
Rej. Dinitrogen oxides. - NH4NO3(s) → N2O(g) + 2H2O(g)
- The gas is soluble in cold water.
- An irritating choking smell of a gas.
- Nitrogen (I) Oxides.
-
- Platinum wire.
- 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
2NO(g) + O2(g) ⇌ 2NO2(g) -
(Accept any 1 correct comparative)Nitrogen (I) Oxide Nitrogen (IV) Oxide. Colourless. Reddish brown. Relights a glowing splint. Extinguishes a glowing splint. Has a sweet smell. Irritating pungent smell. Fairly soluble in water. Readily soluble in water.
-
- It corrodes/reacts with rubber and cork.
-
- Oxidized : Sulphur/S
Reduced: Nitric (V) acid/HNO3(aq) - It decomposes by heat into NO2 which dissolves in the acid.
- Oxidized : Sulphur/S
-
-
- Pass air through purifiers to remove dust particles by electrostatic precipitation. Then pass it through conc. Sodium Hydroxide to absorb CO2. Then through condensers at 25oC to remove water vapour. It is further cooled to liquefy it. The liquefied air is then fractionally distilled to obtain oxygen at – 183oC
-
- X – Ammonia// NH3
Y- Air - 4NO2(g) + 2H2O(s) + O2(g) → 4HNO3(aq)
Accept
2NO2(g) + H2O(l) → HNO3(aq) + HNO2(aq)
2HNO2(aq) + O2(g) → 2HNO3(aq) - Through fractional distillation
- HNO3(aq) + NH3(g) → NH4NO3(aq)
RMM of NH3 = 17 RFM of NH4NO3 = 80
If 80g NH4NO3 = 17 g
960000 =?
960000 x 17 = 2040kg
80 x 1000
- X – Ammonia// NH3
-
- Potassium hydroxide solution
- To remove dust particles
- Water vapour Moisture
- -183oC
- Fractional distillation of liquid air
- Liquid air and passed through fractionating column, where nitrogen with lowest B.P -196oC distils out first and liquid oxygen with highest distil out last.
-
- Nitrogen in liquid form is used as a refrigerant e.g. in storing semen for artificial insemination
- Used as a raw material in Haber process e.t.c - Air is a mixture because:
- It contains gases which are not chemically combined
- The gases are not in fixed ratios.
- Nitrogen in liquid form is used as a refrigerant e.g. in storing semen for artificial insemination
- HOCl(aq) + Dye → HCl (aq) + [Dye + O]
Coloured Colourless √
H2SO3(aq) + [Dye +O] → H2SO4 (aq) + Dye
Coloured Colourless -
- Drying agent √ ½ which must be CaO
Method of collection √ - upward delivery
Workabillity √ ½ - 2NH4Cl(g) + Ca(OH)2(g) → CaCl2(g) + H2O(l) + 2NH3(g) √
- Drying agent √ ½ which must be CaO
-
- Heat
- Cu(g) + N2O(g) → CuO(g) + N2(g)
- - Manufacture of ammonia
- In light bulbs
- As a refrigerant
- – At 113oC consists of S8 rings that flow easily;
- Darkens due to breaking of S8 rings and forming long chains consisting of thousands of atoms. The chains also entangle;
- The long chains consisting of thousands of atoms. The chains also entangle;
- The long chains break near b.p. to form shorter one; - Difference is at the cathode electrode where in concentrated sodium chloride sodium is deposited while in dilute sodium chloride, hydrogen is liberated, because
-
- 2N2O(g) + C(s) → CO2(g) + 2N2(g)
- Ammonium chloride and sodium nitrate
- The hydroxide ions√1 (Ammonia dissolves forming ammonia hydroxide.(1 mk)
-
- E - Ammonium chloride (½ mk)
F – Aluminium hydroxide (½ mk) - Al3+ + 3OH-(aq) → Al(OH)3(s)
- E - Ammonium chloride (½ mk)
-
- Zinc hydroxide
- [Zn(NH3)4 ]2+
- Zn2+(aq) + 2OH(aq) → Zn(OH)2(s)
-
- Plantinum/platinum Rhodium ✓1
- 4NH3(g) + 5O2(g) → 4NO(g) ✓1 + 6H2O(l)
- – Fertilizers ✓1
- Preparation of Nitrogen (I) oxide.
- Explosives
- Blue ppt✓1 is formed which dissolves in excess to form a deep blue ✓1 solution due to formation of tetra amine Copper (II) ions
-
- - Finely divided iron impregnated by alumina (Al2O3)
- 200 atmosphere pressure
- Temperature of 450oC - - CuO is reduced to Copper metal
- NH3 is oxidized to water and nitrogen
- - Finely divided iron impregnated by alumina (Al2O3)
-
- Colour of copper (II) Oxide changes from black to brown
-
- Nitrogen /N2(g)
- Water/H2O(l)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Nitrogen and its Compounds Questions and Answers - Chemistry Form 3 Topical Revision.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students