Questions
- State one observation made in this experiment
- Identify the substances formed in the above reaction
- Hydrogen chloride gas was passed into water as shown below:
- When a blue litmus paper was dropped into the resulting solution, it turned red. Give a reason for this observation
- What is the function of the funnel?
- A group of compounds called chlorofluoro-carbons have a wide range of uses but they also have harmful effects on the environment. State one:-
- Use of chlorofluoro carbons
- Harmful effect of chlorofluoro carbons on the environment.
- Water from a town in Kenya is suspected to contain chloride ions but not sulphate ions.
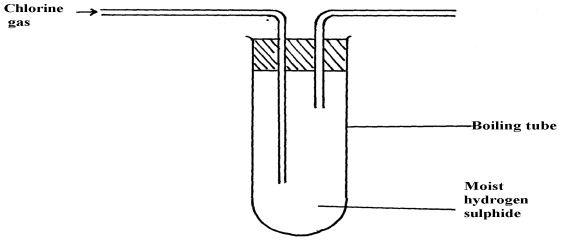
Describe how the presence of the chloride ions in the water can be shown. - In an experiment, chlorine was passed into moist hydrogen sulphide in a boiling tube as shown below:
- What observation was made in the boiling tube?
- Write an equation of the reaction that took place in the boiling tube
- What precaution should be taken in carrying out this experiment? Give a reason
- Heated iron can react with both chlorine gas and hydrogen chloride gas
- Write equations for the reactions
- Chlorine gas has no effect on dry blue litmus paper. Explain
- The following diagram represents a set-up that can be used in the laboratory to prepare and collect a sample of chlorine gas:
- No gas bubbles were produced in the above experiment. Explain the observation
- Complete the following equation
Cl2O(g) + H2O(l) - Describe the bleaching property of chlorine water
- Study the flow diagram below and answer the questions that follow:
- Name gas L ……………………………………………………………
- Write a balanced equation for the reaction between hydrochloric acid and manganese (IV) oxide
- Explain what happens to coloured petals when dropped into a solution of M
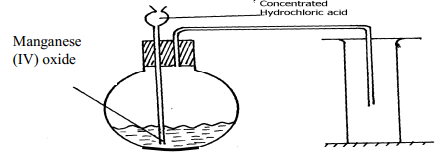
- Two reagents that can be used to prepare chlorine gas are manganese (IV) oxide and concentrated hydrochloric acid.
- Write an equation for the reaction
- Give the formula of another reagent that can be reacted with concentrated hydrochloric acid to produce chlorine gas
- Describe how the chlorine gas could be dried and collected in the laboratory
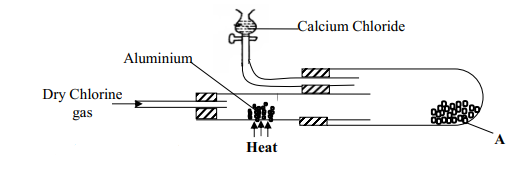
- In an experiment, dry chlorine gas was reacted with aluminium as shown in the diagram below
- Name substance A
- Write an equation for the reaction that took place in the combustion tube
- State the function of the calcium chloride in the set-up above
- Two reagents that can be used to prepare chlorine gas are manganese (IV) oxide and concentrated hydrochloric acid.
- The figure below was set by a student to investigate the reaction between chlorine gas and hydrogen gas:
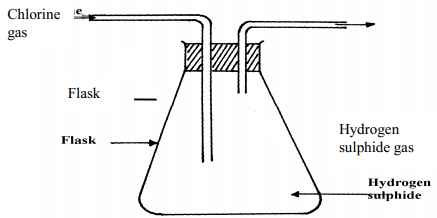
- Write an equation for the reaction that took place in the flask
- What observation was made in the flask?
- What precaution should be taken in carrying out the experiment?
- In an attempt to prepare a gas, Sabulei added concentrated hydrochloric acid to Potassium manganate. The products were then passed through two wash bottles containing water and concentrated sulphuric acid
- Name the gas prepared…………………………………………………………………………
- Name the purpose of wash bottle:
- Containing water?
- Containing concentrated sulphuric acid?
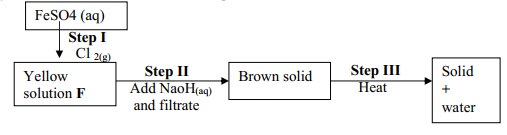
- Study the scheme below and answer the questions that follow.
- Write the formula of the cation present in the yellow solution F
- What property of chlorine is shown in Step II?
- Write an equation for the reaction in step III
- Name one drying agent for hydrogen Chloride
- State and explain the observation that would be made when hydrogen Chloride gas is bubbled into a solution of Silver nitrate
- Carbon (IV) Oxide, methane, nitrogen (I) Oxide and trichloromethane are green house gases
- State one effect of an increased level of these gases to the environment
- Give one source from which each of the following gases is released to the environment;
- Nitrogen (I) Oxide
- Tricholomethane
Answers
-
- It catches fine or presence white fumes
- PCl3 // Phosphorous Trichloride
- PCl5 // Phosphorous Pentachloride
-
- - In water hydrogen chloride dissociates to form hydrogen (H+) and chloride (Cl-) ions.
- The presence of H+ ions in aqueous solution of hydrogen chloride is responsible for acidic properties which turns blue litmus paper red - – To increase the surface area for the dissolution of the gas
- Prevent suck back (Award full 1mk for any one given)
- - In water hydrogen chloride dissociates to form hydrogen (H+) and chloride (Cl-) ions.
-
- – Refrigeration ✓1
- Maintains pressure in aerosol cans and enables sprays tobe sprayed in liquid form - – They deplete the ozone layer. ✓1
- They cause green house effect/Global warming.
- – Refrigeration ✓1
- Acidify water with nitric acid ✓½. Add aqueous lead nitrate/AgNO3 ✓½
Formation of a white ppt. Show presence of Cl- white ppt of PbCl2 or AgCl formed. -
- Yellow solid deposit of sulphur on the wall of boiling tube
- H2S (g) + Cl2 (g) → 2 HCl(g) + S(s)
- - Done in fume chamber/ open air
- Poisonous gases
-
- 2Fe(s) + 3Cl2(g) → 2 FeCl3(g)
Fe(s) + 2HCl(g) → FeCl2(g) + H2(g)
N.B Must be balanced
State symbol must be correct
Chemical symbols must be correct - In the absence of moisture, chlorine cannot form the acidic solution, hence no effect on the blue litmus paper
- 2Fe(s) + 3Cl2(g) → 2 FeCl3(g)
-
- Heat is necessary * REJECT high temperature ACCEPT, BOIL or if implied
- MnO2 is a weak oxidizing agent. - Cl2O(g) + H2O(l) → 2HOCl (aq) C.A.O
- Heat is necessary * REJECT high temperature ACCEPT, BOIL or if implied
-
- Chlorine gas
- HCl(aq) + MnO2(s) → MnCl2(aq) + Cl2(g) + 2H2(g)
- The petals turn to white due to the bleaching effect of NaOCl(sodium hypochlorite)
-
-
- MnO2(s) + 4HCl(l) → MnCl2(aq) + 2H2O + Cl2(g)
Penalize ½mk if state symbols are not correct - KMnO4 or PbO2
- The Chloride gas can be dried by passing it through a wash-bottle of concentrated sulphuric acid and is then collected by downward delivery.
- MnO2(s) + 4HCl(l) → MnCl2(aq) + 2H2O + Cl2(g)
-
- A- Aluminium (III) Chloride
- 2Al(s) + 3Cl2(g) → 2AlCl3(s)
Penalize ½mk for wrong state symbols - Moles of Al used from the equation in b(ii)
= 0.84/27 = 0.031 Moles
Moles of Cl2 used = 0.031 x 3 = 0.047
2
Mark consequently from the equation
-
-
- Cl2(g) + H2S(g) → HCl(g) + S(s)
- Yellow solid particles deposited in the flask
- Excess chlorine and hydrogen sulphide gas should not be emitted into the atmosphere because they are pollutants /harmful ✓ ½
-
- Chlorine gas
-
- Remove traces of hydrogen chloride gas
- Drying agent
-
- Fe3+
- It is an oxidizing agent
- 2Fe(OH)3 (s) → Fe2O3(s) + 3H2O(l)
-
- Anhydrous Calcium Chloride (½mks)
- A white ppt is formed
HCl gas forms Cl- ions solution which react with silver ions to form silver Chloride which is
insoluble OR
HCl(aq) + AgNO3 (aq) → HNO3(aq) + AgCl(s)
Cl-(aq) + Ag+(aq) → AgCl(s)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chlorine and its Compounds Questions and Answers - Chemistry Form 3 Topical Revision.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students