Questions
-
- State the kinetic theory of matter
- State two reasons why gas particles diffuse faster than solid particles
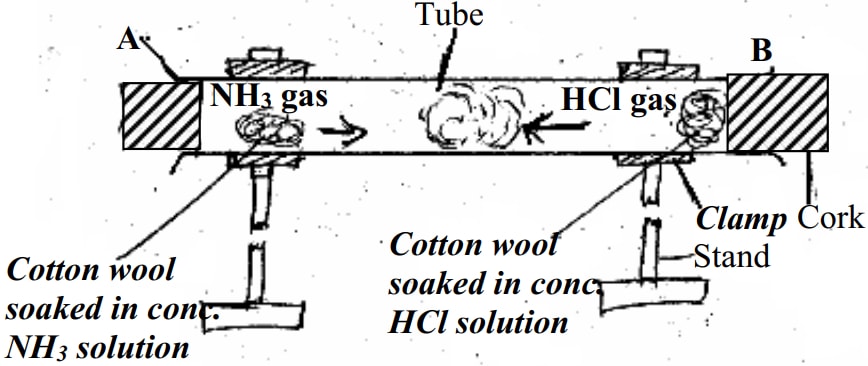
- You are provided with a long glass-tube, fitting corks, cotton wool, concentrated solution hydrochloric acid and concentrated ammonia solution.
- Draw a possible set-up to compare the rates of diffusion of ammonia gas and hydrochloric acid gas
- Outline a clear procedure on how the experiment can be carried out
- What are the possible observations and conclusion
- Distinguish between gases and liquids in terms of inter molecule forces.
- What is the experimental evidence that shows that molecules in gases and liquids are in a state of motion
- State Newton’s second law of motion.
- Smoke particles in air when strongly illuminated were observed to describe continuous, random haphazard movements. Explain what would be observed when the air temperature is decreased
- State how heat transfer by radiation is reduced in a vacuum flask
-
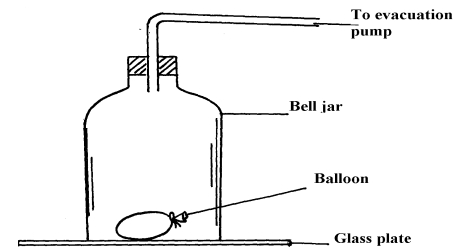
- A partially filled balloon is placed in a bell jar with its open end on a thick glass plate as shown in the figure below. The contact between the jar and the glass plate is greased to make it air tight:
State and explain what happens to the balloon when air in the ball jar is slowly evacuated - The figure below shows an arrangement to demonstrate diffusion through solids:-
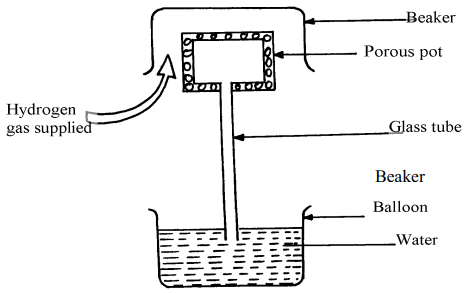
The hydrogen gas is supplied for sometimes then stopped. State and explain what is likely to be observed when the hydrogen gas supply:-- is on
- is stopped
- The diagram below shows a glass tube containing enclosed air by a thread of mercury 50mm long when the tube is held in a horizontal position
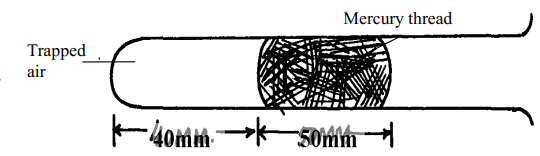
- The tube is slowly raised in a vertical position with the open end facing up. Determine the new length of the trapped air (tube has same area of cross-section; atmospheric pressure = 750mmHg)
- Account for the difference in the column of trapped air using kinetic theory of matter assuming that temperature is constant.
- A partially filled balloon is placed in a bell jar with its open end on a thick glass plate as shown in the figure below. The contact between the jar and the glass plate is greased to make it air tight:
- Two samples of bromine vapour are allowed to diffuse separately under different conditions, one in a vacuum and the other in air. State with reasons the conditions in which bromine will diffuse faster
- In terms of kinetic theory of matter, explain why evaporation causes cooling
-
- In an experiment to demonstrate Brownian motion, smoke was placed in air cell and observed under a microscope. Smoke particles were observed to move randomly in the cell.
- Explain the observation
- Give a reason for using small particles such as those of smoke in this experiment
- What would be the most likely observation if the temperature in the smoke cell was raised?
- An oil drop of average diameter 0.7mm spreads out into a circular patch of diameter 75cm on the surface of water in a trough
- Calculate the average thickness of a molecule of oil
- State two assumptions made in (i) above
- In an experiment to demonstrate Brownian motion, smoke was placed in air cell and observed under a microscope. Smoke particles were observed to move randomly in the cell.
- Give a reason why gases are more compressible than liquids
- Explain the cause of random motion of smoke particles as observed in Brownian motion experiment using a smoke cell.
Answers
-
- The kinetic theory of matter states that matter is made up of tiny particles which are in a constant random motion
- - Gas particles have low cohesive forces
- Gas particles have high kinetic energy
- Gas particles have low density, -
-
- Experiment procedure
- A long glass tube is clamped horizontally as sown in the figure below
- A piece of cotton wool is soaked n concentrated solution of hydrochloric acid and another in concentrated ammonia solution
- Simultaneously, the soaked cotton wool pieces are inserted at the opposite ends of the horizontal glass tube and cork.
- Observations are the noted - Possible observation (1mk for correct observation)
- A white deposit of ammonium chloride forms on the walls on the walls of the glass tube in the region nearer end B
Conclusion (1mk for correct conclusion)
- Different gases have different rates f diffusion
-
- Gases have weaker (small) intermolecular forces while while have relatively stronger (bigger) intermolecular forces ✓1mk Or Water has stronger intermolecular forces than gases. ✓1 mk
- Brownian motion in liquids and gases
- The rare of change of momentum of a body is directly proportional to the resultant external force producing the change and acts in the direction of the force
- The K. E of the smoke particles reduce and hence their movements will be slower (reduces )
- The silver coating ✓ 1 on the inner surfaces of the double walled glass
-
- The balloon expands (increases in volume)
- Evaluation reduces air pressure in the bell jar. Reduction on pressure in the jar leads to expansion of air in balloon -
- Hydrogen gas diffuses faster into the porous pot mixing with air initially in the pot, this increases pressure in the pot causing air to move out through the tube forming bubbles.
- Hydrogen gas diffuses faster out of the pot. This reduces the gas pressure inside the pot hence higher atmospheric pressure on the surface of water in the beaker to push water up the glass tube.
-
- P1V1 = P2V2
P1L1 = P2L2
P1 = 750mmHg, P2 = (750 + 50) = 800mmHg
L1 = 40mm
750 x 40 = 800 x L2
L2 = 750 x 40
800
= 37.5mm - The pressure on trapped air is higher when the tube is vertical than when it is horizontal
Increase in pressure lead to reduction in volume in order to increase the number of collisions per unit time between the air particle and the alls of the glass. This increases the air pressure to balance the increased external pressure.
- P1V1 = P2V2
- The balloon expands (increases in volume)
- Diffusion is faster in vacuum ✓ 1 since there are no air particles to interfere with motion✓ 1
- Energetic molecules gain heat energy from the substance in which the liquid is in contact and escapes. This causes cooling of the latter
-
-
- Air molecules/particles which were in a state of continuous random motion collided with smoke particles
- They are light hence move significantly when bombarded by air molecules
- There would be increased rate of movement
-
- Volume of oil drops = volume f patch
4/3 R3 = 1/4d2t t = thickness
4/3 x (7/2 x 10-4)3 = (0.75)2 t
4
5.7166 x 10-11 = 0.1406t
Thickness, t = 5.7166 x 10-11
0.1406
= 4.066 x 10-10m (accept other units other than metres - Assumptions
- Oil drop forms a perfect sphere (1mk)
- Patch formed is a perfect circle (1mk) (any 2)
- Volume of oil drops = volume f patch
-
- The particles making up gases are further apart than those in liquids ✓
- Smoke particles are constantly bombarded by the air particles which are invisible and in constant random motion
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Particulate Nature of Matter Questions and Answers - Physics Form 1 Topical Revision.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students