INSTRUCTIONS TO CANDIDATES.
- Answer all the questions in the spaces provided below each question.
- All working MUST be clearly shown where necessary.
- Electronic calculators may be used.

Questions
-
- Give two reasons why glass apparatus are used for heating in the laboratory. (2marks)
- The diagram below shows a laboratory apparatus.
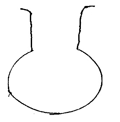
Name the apparatus (1 mark)
-
- State Graham’s law of gaseous diffusion (1 mark)
- 60 cm3 of ozone (O3) diffused through a semi permeable membrane in 80 seconds. Calculate the time taken for 90 cm3 of nitrogen (IV) oxide (NO2) to diffuse under the same conditions. (O=16, N=14). (3marks)
- The grid below is part of the periodic table. Study it and answer the questions that follow. The letters are not actual symbols of elements.
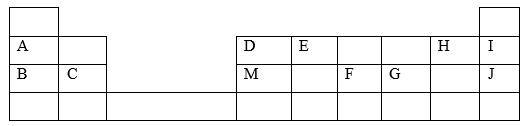
- What is the name given to the chemical family of element C? (1 mark)
- Compare the ionization energies of B and M. Explain. (2 marks)
- Describe how a sample of Lead (II) chloride can be prepared using the following reagents dilute nitric (V) acid; dilute hydrochloric acid and lead carbonate (3marks)
-
- Distinguish between nuclear fission and nuclear fusion. (1 mark)
- Calculate the values of P and Q in the following nuclear equation.
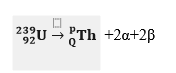 (2 marks)
(2 marks)
-
- When aqueous solution of Iron(II)chloride and potassium thiocyanate are mixed, the equilibrium below was achieved:
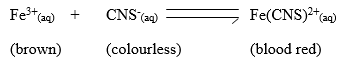
State and explain the effect of adding a few drops of potassium hydroxide to the equilibrium mixture. (2marks) - Given the reaction below
Zn(s) +2HCl(aq) → ZnCl2(aq) +H2(g)
State how the following factors affect the rate of reaction giving explanation- Using Zinc powder instead of granules (1mark)
- Heating the reactants (1mark)
- When aqueous solution of Iron(II)chloride and potassium thiocyanate are mixed, the equilibrium below was achieved:
- Study the flow chart below and answer the questions that follow.
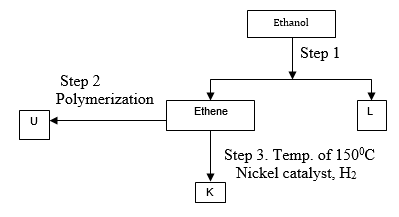
- Identify substances : K, U, L (1 mark)
- State the conditions for the reaction in step 1 to occur. (1 mark)
- The following data refer to an element X.
Calculate the relative atomic mass of element X (2mks)Isotope
P
Q
R
Mass
54
56
57
%abundance
6
92
2
-
- State Faraday’s law of electrolysis (1 mark)
- When a current of 2.5 amperes was passed through a cell containing N2+ ions of a metal for 25minutes, the mass of the cathode increased by 0.36g. Determine the relative atomic mass of element N. (1 Faraday = 96500 coulombs) (3 marks)
- The diagram below represents a set-up used to prepare oxygen gas.
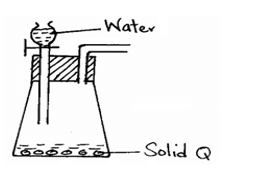
- Name substance Q. (½ mark)
- Complete the set-up to show how dry oxygen gas is collected. (1½ marks)
- Write the equation for the reaction that occurs when magnesium burns in air (1 mark)
- In an experiment to investigate the reaction between acids and metal carbonates, a student reacted zinc carbonate with dilute hydrochloric acid, and the resulting gas was bubbled in excess through lime water in a boiling tube.
- State and explain the observations made in the boiling tube at the end of the experiment. (2 marks)
- Write an equation for the reaction took place at the end of the experiment. (1 mark).
- Use the thermo-chemical equations below to answer the questions that follow.
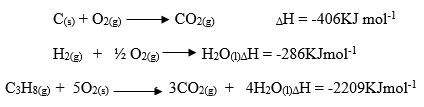
Draw an energy cycle diagram and use it to calculate the heat of formation of propane. (3 marks) - A student mixed equal volumes of Ethanol and ethanoic acid. He added a few drops of concentrated Sulphuric (VI) acid and warmed the mixture.
- Name and write the formula of the main products (1 mark)
Name…………………………………………………………………..…….
Formula…………………………………………………………………..….. - Which homologous series does the product named in (i) above belong? (1mark)
- Name and write the formula of the main products (1 mark)
- The apparatus below was set up to show the catalytic oxidation of ammonia. Study the diagram and answer the questions that follow
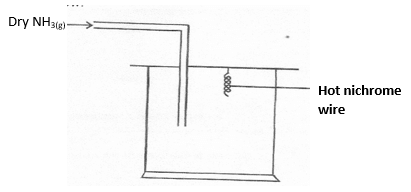
- Write an equation for the reaction that takes place in the gas jar (1mark)
- What is the role of hot nichrome wire? (1mark)
- Write the formula of the complex ion formed when excess ammonia gas is passed through a solution containing Zn2+ ions. (1mark)
-
- Define the term allotrope (1mark)
- Name two non-crystalline allotropes of sulphur (1mark)
- Diamond and graphite are both allotropes of carbon. Explain why graphite is used as a lubricant whereas diamond is used as an abrasive. (1 mark)
- The table below shows PH values of some solutions
Solution
A
B
C
D
PH values
13
7
1
6.5
- What solution reacts vigorously with Magnesium metal? (1mark)
- Industrial Hydrochloric acid (1mark)
- Which solution forms complex ions with zinc (II) oxide? (1mark)
- Draw the structural formula for each of the following compounds
- 4,4-dimethylpent-2-ene (1mark)
- Give the systematic IUPAC name of the following substances
- CH3 CH Br CH Br CH3 (1mark)
- A hydrated salt has the following composition by mass. Iron is 20.2%, oxygen is 23.0% sulphur is 11.5%, water 45.3%. Its relative formula mass is 278. Determine the formula of the hydrated salt.
(Fe=56, S=32.0, O=16, H=1) (2marks) - The diagram shows the apparatus used to separate different dyes in food colouring.
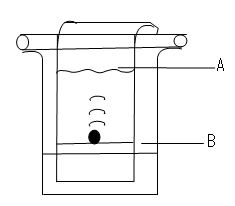
- Name the parts labeled A & B (1 mark)
A: …………………………………………………………………………………………
B: ……………….………………………………………………………………………… - Thediagram below shows electrolysis of lead bromide
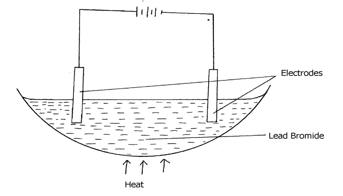
- Label the anode and cathode (1mark)
- Write half equations to shows reactions at cathode. (1mark)
- Name the parts labeled A & B (1 mark)
- The set-up below shows the products formed when solid lead (ii) nitrate is heated.
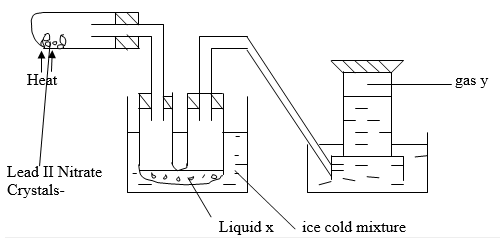
- Identify:
- Liquid x …………………………………………………………… (½ mark)
- Gas y……………………………………………………………… (½ mark)
- Write an equation for the reaction taking place in the combustion tube. (1 mark)
- Identify:
- Study the flow chart below and answer the questions that follow.
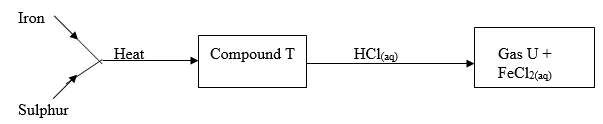
- Name:
- Compound T _________________________________________ (½ mark)
- Gas U ______________________________________________ (½ mark)
- State a physical property that you could use to identify gas U. (1 mark)
- Name:
-
- The melting point of phosphorous trichloride is -918º C and that of sodium chloride is 801º C. Explain the huge difference in their melting points. (2marks)
- The electronic arrangement of two stable ions Q2+ and P2- are 2.8.8 and 2.8.8 respectively.
- Write the electron arrangement of neutral atoms Q and P. (1 mark)
Q………………………………………………………………………………………....…
P………………………………………………………………………………………....… - What is the most likely structure of an oxide element P? (1 mark)
- Write the electron arrangement of neutral atoms Q and P. (1 mark)
- The structures shown below represents two cleansing agents A and B.
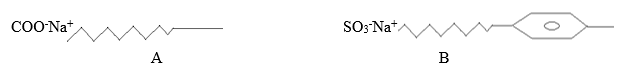
- Identify the cleansing agents A and B (1 mark)
A ……………………………………………………………………………………………………
B ……………………………………………………………………………………………………. - Explain how the cleaning properties of the above cleansing agents can be improved. (1 mark)
- Identify the cleansing agents A and B (1 mark)
- Sulphur burns in air to form sulpur (IV) oxide. A simple energy level energy level diagram for the reaction is given below. Study the diagram and answer the questions that follow:
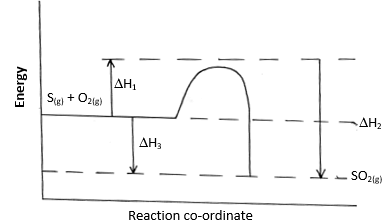
- On the diagram indicate the activation energy (1 mark)
- Write an expression for ΔH3 in terms of ΔH1 and ΔH2 (1mark)
- Study the flow chart below and use it to answer the questions that follow:
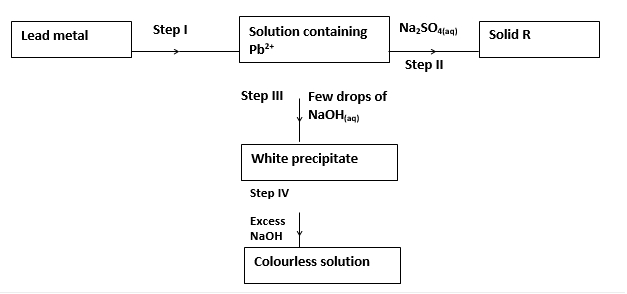
- Identify the reagent used in step I. (1mark)
- Name solid R. (1mark)
- Explain the observation in step IV. (1mark)
- Study the set- up below and answer questions that follows.
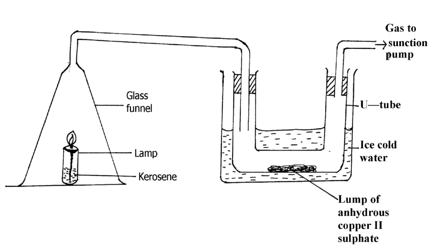
- State and explain the observation made in the U- tube. (1½ marks)
- Explain what will happen to lamp when the suction pump is turned off. (1½ marks)
- Using dot ( •) and crosses ( x) diagram to represent electrons in the outer most energy levels only show bonding in;
- Phosphine molecule.PH+4. (P = 15, H = 1) (1 mark)
- Ammonia (NH3) (1 mark)
- The set up below was used to prepare dry sample of chlorine gas.
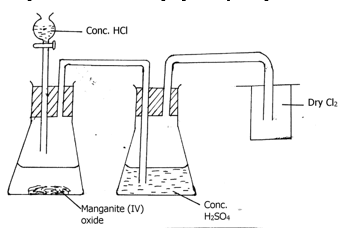
- What is the function of manganese (IV) oxide in the preparation of chloride (1mark)
- Write a chemical equation for the formation of chlorine gas. (1 mark)
- Explain the observations made when chlorine gas is bubbled through a solution of iron II sulphate. (2marks)







Marking Scheme
-
- Give two reasons why glass apparatus are used for heating in the laboratory. (2 marks)
- Have high m.p and b.p
- Are good conductors of heat
- The diagram below shows a laboratory apparatus.
Name the apparatus (1 mark)- Round-bottomed flask ✓ 1½
- Give two reasons why glass apparatus are used for heating in the laboratory. (2 marks)
-
- State Graham’s law of gaseous diffusion (1 mark)
- The rate of diffusion of a gas is inversely proportional to the square root of its density under the same conditions of temperature and pressure.
- 60 cm3 of ozone (O3) diffused through a semi permeable membrane in 80 seconds. Calculate the time taken for 90 cm3 of nitrogen (IV) oxide (NO2) to diffuse under the same conditions. (O=16, N=14). (3mks)
- 60cm3 of ozone takes 80seconds
90cm3 of ozone takes 120seconds
NO2 =56 O3 = 48
TNO2 = √RMM. NO2
TO3 √RMM. O3
TNO2 = 120√56
√48
= 129.6 seconds
- 60cm3 of ozone takes 80seconds
- State Graham’s law of gaseous diffusion (1 mark)
- The grid below is part of the periodic table. Study it and answer the questions that follow. The letters are not actual symbols of elements.
- What is the name given to the chemical family of element C? (1 mark)
- Alkaline earth metals
- Compare the ionization energies of B and M. Explain. (2 marks)
- M has higher ionization energy than B
- M has smaller atomic radius with a stronger nuclear force of attraction thus more energy is required to remove an electron in its outermost energy level.
- What is the name given to the chemical family of element C? (1 mark)
- Describe how a sample of Lead (II) chloride can be prepared using the following reagents dilute nitric (V) acid; dilute hydrochloric acid and lead carbonate (3mks)
- React excess lead carbonate with dilute nitric (v) acid√ ½ . Filter √½ to obtain lead nitrate as filtrate ; react the filtrate with dilute hydrochloric acid ; a precipitate of lead (ii) chloride is formed ; wash the residue of pbCl2 and dry between filter papers.
-
- Distinguish between nuclear fission and nuclear fusion. (1 mark)
- Nuclear fission is the splitting process a heavy nuclide undergoes when bombarded by a fast-moving neutron while nuclear fusion occurs when nuclei combine together when they are made to collide at high velocity resulting in the formation of a heavy nucleus and release of energy.
- Calculate the values of P and Q in the following nuclear equation.
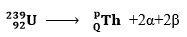 (2 marks)\
(2 marks)\
239 = p + (2×4) + 0 ✓½
p =239 – 8.
P = 231 ✓½
92 = Q + (2×2) + (2× −1) ✓½
92 = Q + 2
Q = 92 – 2
Q = 90 ✓½
- Distinguish between nuclear fission and nuclear fusion. (1 mark)
-
- When aqueous solution of Iron(II)chloride and potassium thiocyanate are mixed, the equilibrium below was achieved:
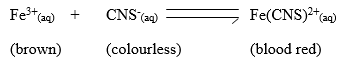
State and explain the effect of adding a few drops of potasium hydroxide to the equilibrium mixture. (2marks)- Equilibrium will shift to the ½ left/ backward reaction favoured // more brown will be formed OH- ions precipitates Fe3+ ions in form of Fe(OH)3
- Given the reaction below
Zn(s) +2HCl(aq) → ZnCl2(aq) +H2(g)
State how the following factors affect the rate of reaction giving explanation- Using Zinc powder instead of granules (1mk)
- Increase the rate of reaction. Because it increases the surface area of contact with hydrochloric acid.√1
- Heating the reactants (1mk)
- Increases the rate of reaction because increase in temperature increase kinetic energy resulting in higher frequency of collision of particles.√1
- Using Zinc powder instead of granules (1mk)
- When aqueous solution of Iron(II)chloride and potassium thiocyanate are mixed, the equilibrium below was achieved:
- Study the flow chart below and answer the questions that follow.
- Identify substances : K, U, L (1 mark)
- K – Ethane ✓½
- U - Polythene ✓½
- L – Water
Any two correct answers
- State the conditions for the reaction in step 1 to occur. (1 mark)
- Temperature – 1800C ✓½
- Conc. Sulphuric vi acid ✓½
- Identify substances : K, U, L (1 mark)
- The following data refer to an element X.
Calculate the relative atomic mass of element X (2mks)Isotope
P
Q
R
Mass
54
56
57
%abundance
6
92
2
- (54×6) + (56×92) + (57×2)
100
= 55.9 ✓½
- (54×6) + (56×92) + (57×2)
-
- State Faraday’s law of electrolysis (1 mark)
- The mass of a substance produced during electrolysis is directly proportional to the quantity of electricity passed.
- When a current of 2.5 amperes was passed through a cell containing N2+ ions of a metal for 25minutes, the mass of the cathode increased by 0.36g. Determine the relative atomic mass of element N. ( 1 Faraday = 96500 coulombs) (3mks)
- Q = It
Q = 2.5 x 25 x 60 = 3750C ✓ ½
N2+ - 2 faradays ✓ ½
1 faraday – 96500C x 2 = 193000 ✓ ½
3750C = 0.36g
193000 C = ?
therefore: 0.36 x 193000
3750
= 18.528
= 18.5 ✓ ½
- Q = It
- State Faraday’s law of electrolysis (1 mark)
- The diagram below represents a set-up used to prepare oxygen gas.
- Name substance Q. (½ mark)
- Sodium Peroxide
- Complete the set-up to show how dry oxygen gas is collected. (1½ marks)
- Write the equation for the reaction that occurs when magnesium burns in air (1 mark)
- 2 Na2O2 + 2 H2O → 4NaOH + O2
(aq) (l) (aq) (g)
- 2 Na2O2 + 2 H2O → 4NaOH + O2
- Name substance Q. (½ mark)
- In an experiment to investigate the reaction between acids and metal carbonates, a student reacted zinc carbonate with dilute hydrochloric acid, and the resulting gas was bubbled in excess through lime water in a boiling tube.
- State and explain the observations made in the boiling tube at the end of the experiment (2 marks)
- The white precipitate dissolves to form a colourless solution. 1 This is due to formation of soluble calcium hydrogen carbonate. 1
- Write an equation for the reaction took place at the end of the experiment. (1 mark).
- CaCO3(s) + H2O(l) + CO2(g) → Ca(HCO3)2(aq)
- State and explain the observations made in the boiling tube at the end of the experiment (2 marks)
- Use the thermo-chemical equations below to answer the questions that follow.
C(s) + O2(g) → CO2(g) ΔH = -406KJ mol-1
H2(g) + ½ O2(g) H2O(l) ΔH = -286KJmol-1
C3H8(g) + 5O2(s) 3CO2(g) + 4H2O(l) ΔH = -2209KJmol-1
Draw an energy cycle diagram and use it to calculate the heat of formation of propane. (3 marks)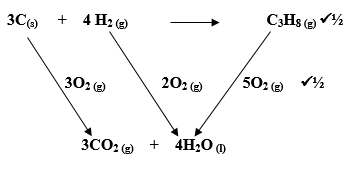
ΔHf = [3(-406) + 4(-286)] – [-2209]
= [-1218 – 1144] + [2209]
= -153 KJmol-1 - A student mixed equal volumes of Ethanol and ethanoic acid. He added a few drops of concentrated Sulphuric (VI) acid and warmed the mixture.
- Name and write the formula of the main products (1 mark)
- Name Ethylethanoate
- Formula CH3COOCH2CH3
- Which homologous series does the product named in (i) above belong? (1mk)
- Esters
- Name and write the formula of the main products (1 mark)
- The apparatus below was set up to show the catalytic oxidation of ammonia. Study the diagram and answer the questions that follow
- Write an equation for the reaction that takes place in the gas jar (1mk)
- 4NH3(g) + 5O2 (g) 4NO(g) + 6H2O(g)
- What is the role of hot nichrome wire? (1mk)
- Hot nichrome wire catalyses the reaction between ammonia and oxygen / oxidation of NH3√1.
- Write the formula of the complex ion formed when excess ammonia gas is passed through a solution containing Zn2+ ions. (1mk) 2+
- [Zn(NH3)4]2+ ✓1
- Write an equation for the reaction that takes place in the gas jar (1mk)
-
- Define the term allotrope (1mk)
- Existence of an element in different forms but in the same physical state.
- Name two non-crystalline allotropes of sulphur (1mk)
- Rhombic
- Monoclinic
- Diamond and graphite are both allotropes of carbon. Explain why graphite is used as a lubricant whereas diamond is used as an abrassive . (1 mark)
- Van der waal forces in graphite white in diamond there is strong covalent bonds
- Define the term allotrope (1mk)
- The table below shows PH values of some solutions
Solution
A
B
C
D
PH values
13
7
1
6.5
- What solution reacts vigorously with Magnesium metal? (1 mark)
- C
- Industrial Hydrochloric acid (1 mark)
- C
- Which solution forms complex ions with zinc (II) oxide? (1 mark)
- C
- What solution reacts vigorously with Magnesium metal? (1 mark)
- Draw the structural formula for each of the following compounds
- 4,4-dimethylpent-2-ene (1mk)
-
- Give the systematic IUPAC name of the following substances
- CH3 CH Br CH Br CH3 (1mk)
- 2, 3 – dibromobutane
- A hydrated salt has the following composition by mass. Iron is 20.2%, oxygen is 23.0% sulphur is 11.5%, water 45.3%. Its relative formula mass is 278. Determine the formula of the hydrated salt.
(Fe=56, S=32.0, O=16, H=1) (3mks)
Fe O S H2O
Mass 20.2 23.0 11.5 45.3
R.A.M 56 16 32 18
Moles 0.36 1.44 0.36 2.52
Mole Ratio 1 : 4 : 1 : 7
Empirical Formula FeSO4.7H2O Molecular formula FeSO4.7H2O - The diagram shows the apparatus used to separate different dyes in food colouring.
- Name the parts labeled A & B (1 mark)
- A: Solvent front
- B: Baseline
- The diagram below shows electrolysis of lead bromide
- Label the anode and cathode (1mk)
- On the diagram ( left hand electrode – Anode, right hand electrode – Cathode)
- Write half equations to shows reactions at cathode. (1mk)
- Pb2+(l) + 2e- → Pb(s)
- Label the anode and cathode (1mk)
- Name the parts labeled A & B (1 mark)
- The set-up below shows the products formed when solid lead (ii) nitrate is heated.
- Identify:
- Liquid x …………………………………………………………… (½ mark)
- Dinitrogen tetraoxide / N2O4 ✓½
- Gas y……………………………………………………………… (½ mark)
- Oxygen/ O2 ✓½
- Liquid x …………………………………………………………… (½ mark)
- Write an equation for the reaction taking place in the combustion tube. (1 mark)
- Identify:
- Study the flow chart below and answer the questions that follow.
- Name :
- Compound T
- Iron (II) sulphide ✓½
- Gas U
- Hydrogen sulphide ✓½
- Compound T
- State a physical property that you could use to identify gas U. (1 mark)
- Gas has a characteristic smell of rotten eggs
- Name :
-
- The melting point of phosphorous trichloride is -918º C and that of sodium chloride is 801º C. Explain the huge difference in their melting points. (2mks)
- PCl3 has simple molecular structure held by weak van der waals forces wit require less energy to break. While NaCl has strong ionic bonds with giant ionic structure that require more energy to break.
- The melting point of phosphorous trichloride is -918º C and that of sodium chloride is 801º C. Explain the huge difference in their melting points. (2mks)
- The electronic arrangement of two stable ions Q2+ and P2- are 2.8.8 and 2.8.8 respectively.
- Write the electron arrangement of neutral atoms Q and P. (1 mark)
- Q – 2.8.8.2
- P – 2.8.6
- What is the most likely structure of an oxide element P? (1 mark)
- Simple molecular structure
- Write the electron arrangement of neutral atoms Q and P. (1 mark)
- The structures shown below represents two cleansing agents A and B.
- Identify the cleansing agents A and B (1 mark)
- A – soapy detergent
- B – soapless detergent
- Explain how the cleaning properties of the above cleansing agents can be improved. (1 mark)
- Tetraoxophosphate materials/compounds are added to the cleansing agents. The compounds prevent formation of compounds with Ca2+, Mg2+ ions hence no scum is formed.
- Identify the cleansing agents A and B (1 mark)
- Sulphur burns in air to form sulpur (IV) oxide. A simple energy level energy level diagram for the reaction is given below. Study the diagram and answer the questions that follow:
- On the diagram indicate the activation energy (1 mark)
- Write an expression for ΔH3 in terms of ΔH1 and ΔH2 (1 mark)
- ΔH3 = ΔH1 - ΔH2
- Study the flow chart below and use it to answer the questions that follow:
- Identify the reagent used in step I. (1mk)
- Dilute Nitric (V) acid
- Name solid R. (1mk)
- Lead (II) Sulphate
- Explain the observation in step IV. (1mk)
- Amphoteric lead hydroxide reacts with basic sodium hydroxide forming a complex ion. (tetrahydroxo lead II ions) which are colourless.
- Identify the reagent used in step I. (1mk)
- Study the set- up below and answer questions that follows.
- State and explain the observation made in the U- tube. (1½ marks)
- Colour of anhydrous copper II sulphate changes from white to blue . The kerosene lamp burns to produce CO2 and H2O water combines with anhydrous copper II sulphate (white ) to form hydrated copper (II) sulphate blue.
- Explain what will happen to lamp when the sunction pump is turned off. (1½ marks)
- The lamp goes off .This is due to accumulation of CO2 which is denser than air.
- State and explain the observation made in the U- tube. (1½ marks)
- Using dot ( •) and crosses ( x) diagram to represent electrons in the outer most energy levels only show bonding in;
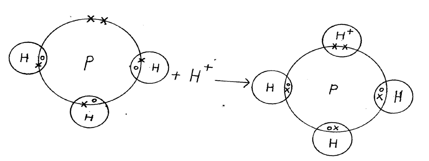
- Phosphine molecule.PH+4.( P = 15 , H = 1) (1 mark)

- Ammonia (NH3) (1 mark)
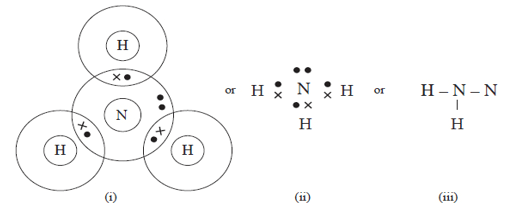
- Phosphine molecule.PH+4.( P = 15 , H = 1) (1 mark)
- The set up below was used to prepare dry sample of chlorine gas.
- What is the function of manganese (IV) oxide in the preparation of chloride (1 mark)
- It is an oxidizing agent.
- Write a chemical equation for the formation of chlorine gas. (1 mark)
- Explain the observations made when chlorine gas is bubbled through a solution of iron II sulphate. (2 marks)
- The colour of iron II sulphate changes from pale green to red brown.
- Chlorine gas oxidizes Iron II ions to Iron III ions
- What is the function of manganese (IV) oxide in the preparation of chloride (1 mark)
Download Chemistry Paper 1 Questions and Answers - KCSE 2022 Prediction.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

