- Give the name of the first member of the alkene homologous series.
ethene -
When a few drops of aqueous ammonia were added to copper (II) chloride solution, a pale blue precipitate was formed. On addition of excess aqueous ammonia, a deep blue solution was formed. Identify the substance responsible for the
a. Pale blue precipitate
b. Deep blue precipitateThe copper ion in the aqueous solution of exists predominantly as [Cu(H2O)6]2+. This complex ion imparts a characteristic pale blue color to the solution.
Continued addition of ammonia results the formation of a soluble deep blue complex copper ammonia ion: -
30cm3 of carbon (II) oxide diffuses in 30 seconds. How long will 60cm3 of nitrogen gas take to diffuse under the same conditions.
( C = 12, O = 16, N = 14)Graham's law of diffusion
rate1/rate2 = √m2/√m1
mass of carbon (II) oxide = 12 + 16 + 16 = 44
30/x = √44/√14 -
Hardness in water is caused by dissolved salts.
Which cations cause water hardness.
Ca2+ Mg2+ - Name isomers of butane.
n-butane and 2-methylpropane - Name 2 allotrobes of carbon
graphite and diamond - State the bonds present in both allotropes
graphite: weak van-der-waals
diamond: strong covalent bonds - The diagram below shows some properties of ammonia gas. Use it to answer the questions that follow.
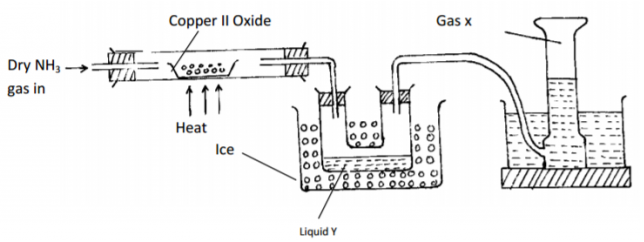
State the observation made in the combustion tube.
Copper II oxide changes from black to brown as it is reduced.
Give the test that can be used to identify liquid Y.
it turns blue cobalt(ii)chloride to pink, or white copper (II) sulphate blue - What is vulcanization of rubber?
Vulcanization or vulcanisation is a chemical process for converting natural rubber or related polymers into more durable materials by the addition of sulfur or other equivalent curatives or accelerators. These additives modify the polymer by forming cross-links (bridges) between individual polymer chains. -
A piece of burning Magnesium was introduced into a jar of nitrogen.
State what was observed.
A white ash was deposited - Write an equation for the reaction that took place.
3Mg + N2 --> Mg3N2 - Zinc reacts with both concentrated and dilute sulphuric (VI) acid. Write equations for the two reactions.
Dilute: Zn + H2SO4 = ZnSO4 + H2
Conc: Zn + 2H2SO4 -----> ZnSO4 + SO2 + 2H2O
Download Chemistry MOCK revision with answers 30/9/16.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

