- The set up below can be used to prepare oxygen gas. Study it and answer the questions that follow.
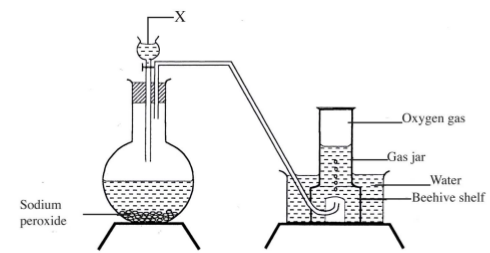
- Identify X. (1 mark)
- What property of oxygen makes it possible for it to be collected as shown in the above set up? (1 mark)
- State two uses of oxygen gas. (1 mark)
- Write equations to show the effect of heat on each of the following:
- Sodium hydrogen carbonate; (1 mark)
- Silver nitrate; (1 mark)
- Anhydrous iron (II) sulphate. (1 mark)
- Describe an experiment procedure that can be used to extract oil from nut seeds. (2 marks)
- In terms of structure and bonding, explain the following observations:
- The melting point point of aluminium is higher than that of sodium. (1 mark)
- Melting point of chlorine is lower than that of sulphur. (1 mark)
- The diagram below illustrates a method of preparing salts by direct synthesis.
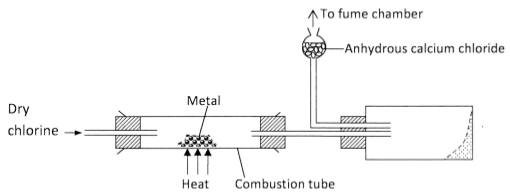
- This method can be used to prepare either aluminium chloride or iron (III) chloride. Explain why it cannot be used to prepare sodium chloride. (1 mark)
- Describe how a sample of sodium chloride can be prepared in the laboratory by direct synthesis. (2 marks)
-
- A student electroplated a spoon with copper metal. Write an equation for the process that took place at the cathode. (1 mark)
- Calculate the time in minutes required to deposit 1.184g of copper if a current of 2 amperes was used. (1 Faraday = 96500 coulombs, Cu = 63.5) (2 marks)
- Study the flow chart below and answer the questions that follow:
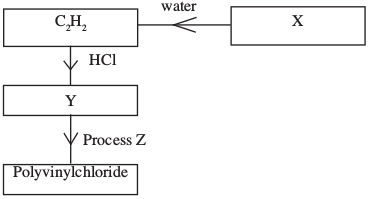
- Identify:
- X (1 mark)
- Y (1 mark)
- State two uses of polyvinylchloride. (1 mark)
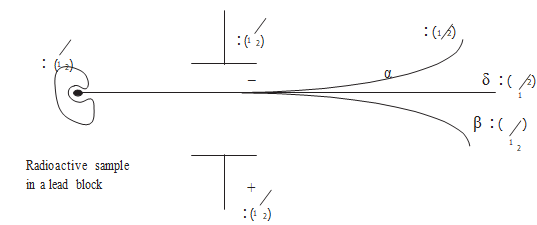
- Identify:
- Draw a labeled diagram to illustrate how alpha, beta and gamma radiation can be distinguished from each other. (3 marks)
- Aqueous hydrogen chloride reacts with potassium manganate (IV) to produce chlorine gas, while a solution of hydrogen chloride in menthylbenzene has no effect on potassium manganate (VII). Explain this observation. (2 marks)
- The table below gives the solubilities of substances T and U at 100C and 400C.
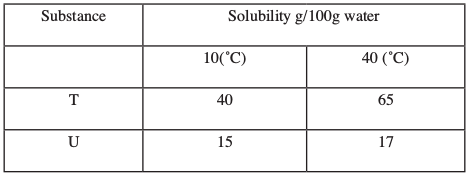
When an aqueous mixture containing 55 g of T and 12 g of U at 80oC was cooled to 10oC, crystals were formed.- Identify the crystals formed. (1 mark)
- Determine the mass of the crystals formed. (1 mark)
- Name the method used to obtain the crystals. (1 mark)
- Hydrazine gas,
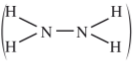 burns in oxygen to form nitrogen gas and steam.
burns in oxygen to form nitrogen gas and steam.- Write an equation for the reaction. (1 mark)
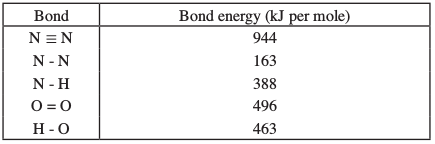
- Using the bond energies given below, calculate the enthalpy change for the reaction in (a) above. (2 marks)
-
- What would be observed if sulphur (IV) oxide is bubbled through acidified potasium manganate (VII)? (1 mark)
- In an experiment, sulphur (IV) was dissolved in water to form solution L.
- What would be observed if a few drops of barium nitrate solution were immediately added to the solution L? (1 mark)
- Write an ionic equation for the reaction that occured between solution L and barium aqueous nitrate in (b)(i) above. (1 mark)
- The scheme below shows some reaction sequence starting with solid N. Study it and answer the questions that follow.
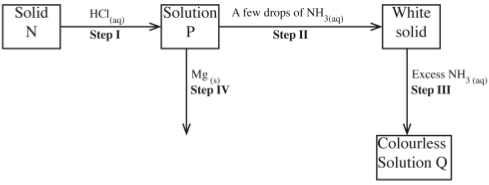
- Write the formula of the complex ion is solution Q. (1 mark)
- Write an equation for the reaction in step IV. (1 mark)
-
- State Charles' Law. (1 mark)
- A certain mass of gas occupies 146 dm3 at 291 K and 98.31 kPa.
What wil be its temperature if its volume is reduced to 133 dm3 at 101.32 kPa? (2 marks)
- The chromatogram below was obtained from a contaminated food sample P. Contaminants Q, R, S and T are suspected to be in P. Use it to answer the following questions.
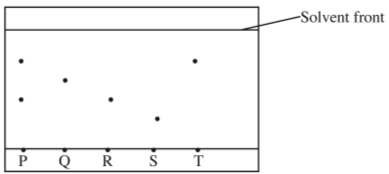
- Identify the contaminants in mixture P. (1 mark)
- Which is the most soluble contaminant in P? (1 mark)
- The curves below represent the change in mass when equal masses of powdered zinc and zinc granules were reacted excess 2M hydrochloric acid. Study them and answer the question below.
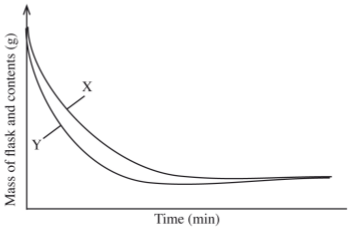
Which curve represents the reaction with zinc granules? Explain your answer. (3 marks) - When fuels burn in the internal combustion engine at high temperature, one of the products formed is nitrogen(II) oxide.
- Write an equation for the formation of nitrogen (II) oxide. (1 mark)
- Give a reason why nitrogen(II) oxide is not formed at room temperature. (1 mark)
- Describe how formation of nitrogen (II) oxide i the internal combustion engine leads to gaseous pollution. (1 mark)
- The set up below was used to investigate the products of burning biogas (methane). Study it and answer the questions that follow.
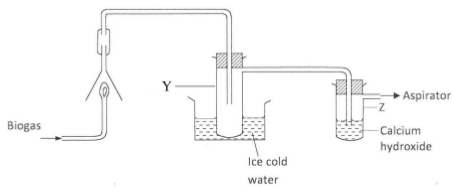
- What product will be formed in test-tube Y? (1 mark)
- State and explain the observation which would be made in Z. (2 marks)
-
- Diamond and graphite are allotrophes of carbon. What is meant by an allotrope? (1 mark)
- Explain why graphite can be used as a lubricant while diamond cannot. (2 marks)
- The plots below were obtained when the atomic radii of some elements in group I and II were plotted against atomic numbers.
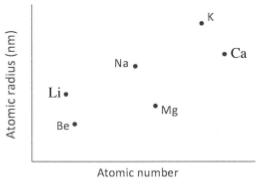
Explain:- The trend shown by Li, Na and K. (1 mark)
- why the atomic radii of elements Be, Mg and Ca are lower than those of Li, Na and K. (2 marks)
- On heating a pale green solid K, carbon (IV) oxide gas and a black solid M were formed. On reacting K with dilute hydrochloric acid, carbon (IV) oxide gas and a green solution S were formed. When excess aqueous ammonia was adde dto solution S, a deep blue solution was formed.
- Identify the cation in solid K. (1 mark)
- Identify two anions in soluion S. (2 marks)
-
- Name two ores from which copper is extracted. (1 mark)
- During extraction of copper metal, the ore is subjected to froth flotation. Give a reason why this process is necessary. (1 mark)
- Name one alloy of copper and state its use. (1 mark)
- When 15cm3 of gaseous hydrocarbon, P, was burnt in 100cm3 of oxygen, the resuting gaseous mixture occupied 70cm3 at room temperature and preassure.When the gaseous mixture was passed through potassium hydroxide solution, its volume decreased to 25cm3.
- What volume of oxygen was used during the reaction? (1 mark)
- Determine the molecular formular of the hydrocarbon. (2 marks)
- A solution was made by dissolving 8.2g of calcium nitrate to give two litres of solution.
(Ca = 40.0; N = 14.0; O = 16). (3 marks)
Determine the concentration of nitrate ions in moles per litre. (3 marks) - State and explain what would happen if a dry red litmus paper was dropped in a gas of dry chlorine. (2 marks)
- By using aqueous sodium chloride, describe how a student can distinguish calcium ions from lead ions. (2 marks)
- A student investigated a property of acids M and N by reacting equal volumes of acid M and N of the same concentration with equal volumes of 2M potassium hydroxide. The results were recorded in the table below.

- Which of the acids is likely to be a weak acid? Explain. (2 marks)
- Write the equation for the reaction between ethanoic acid and potassium hydroxide. (1 mark)
- A student investigated the effect of an electric current by passing it through some substances. The student used inert electrodes, and connected a bulb to the circuit. The table below shows the substances used and their states.

- In which experiments did the bulb not light? (1 mark)
- Explain your answer in (a) above. (2 marks)
- A sample of hydrogen gas was found to be a mixture of two isotopes, 11H and 21H.
Determine the relative molecular masses of the molecules formed , when each of these isotopes is burnt in oxygen. (O = 16.0)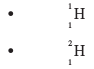 (2 marks)
(2 marks)

MARKING SCHEME
-
- X is water. or H2O
- It is slightly soluble in water. and denser than air.
-
- Used in hospitals to resuscitate patients.
- Used in welding when mixed with acetylene in the ocy-acetylene flame.
- Used by divers and mountaineers.
- Rocket fuel, hospitals for breathing, steel making.
-
- 2NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(g)
- 2AgNO3(s) → Ag(s) + 2NO2(g) + O2(g)
- 2FeSO4(s) → Fe2O3(s) + SO2(g) + SO3(g)
-
- Crush the seeds in a mortar using a pestle.
- Add a suitable solvent (acetone / propanone
- Filter out the solid matter.
- Evaporate the filterate to obtain oil.
-
- Aluminium has a stronger metallic bond because it has more delocalised electrons
- Sulphur has a ringed structure of S8 molecules whiles chlorine is diatomic. The forces in sulphur are stronger than chlorine.
-
- It does not sublime.
- Cut a piece of Sodium metal, place it on a deflagrating spoon, heat it briefly
then lower it into a gas jar of chlorine . It will continue burning forming
Sodium Chloride.
-
- Cu2+(aq) + 2e → Cu(s)
- 63.5 g require 2 x 96500 C
1.184g = 2 x 96500 x 1.184
63.5
3598.6 coulombs : (1)
Q = 1t 1799.2
60
3586.5 = 2 x t
3586.5 = t = 29.988
2
- 30 minutes
1799.3 s = t
-
-
- X - Calcium carbide or CaC2
- Y - CH2 = CHCl Chloroethene or vinylchloride
- Floor tiles
Rain coats Any 2
-
-
(Accept any other working diagram)
Working diagram, α should be deflected less than β because of its heavier mass. - In water, HCl is ionised into H+ and Cl- the Chloride ions are oxidised to chlorine gas by potassium permanganete.
In methylbenzene, HCl remains in molecular form i.e HCl. The Chloride is not available for oxidation hence no reaction. -
- T(1)
- 15 g
- Fractional crystallization
-
- N2H4(g) + O2(g) → N2(g) + 2H2O(g)
- Bond breaking energy
163 + 4 (388) + 496
= 2211 kJ
Bond making energy
944 + 4 (463)
2796 kJ
Ethalpy change = Bond breaking + Bond making energies.
2211 + (-2796)
= -585 kJ/mol
-
- The acidified permanganete will be decolourised (purple to colourless)
The permanganate (VII) is reduced to manganese (II) ion. -
- A white precipitate forms.
- Ba2+(aq) + SO32-(aq) → BaSO3(s)
- The acidified permanganete will be decolourised (purple to colourless)
-
- [Zn(NH3)] 2 + 4 :(1)
- [Z2+(aq) + Mg(s) → Zn(s) + Mg2+(aq) ZnCl(2)(aq) + Mg(s) → Zn(s) + MgCl2(aq)
-
- Charles Law
At constant pressure, the volume of a fixed mass of gas is directly proportional to its absolute temperature. - P1V1 = P2V2
T1 T2
T2 = P2V2T1
P1V1
T2 =100 x 133 x 361
98.39 x 146
T2 =4849313
14364.94
T2 = 273.22 K
P1 = 98.39kPa
V1 = 146dμ3
T1 = 18 + 273 = 361K
P2 = 101kPa
V2 = 133
t2 = ?
- Charles Law
-
- R and T
- T
- X- Zinc granules
The gradient of the graph is less steep because there is less surface area. -
- N2(g) + O2(g) → 2NO(g)
- Because nitrogen is inert.
- Nitrogen (II) oxide is oxidised to Nitrogen (IV) oxide which is a pollutant.
-
- Water
- Bubbles of gas : (1 2) and a white ppt
CO2. reacts to give CaCO3
-
- These are different forms carbon in the same physical state.
- The hexagonal graphite rings have weak Van der Waals forces between the layers that allow the layers to slide over each other while in diamond the atoms are held by strong Covalent bonds.
-
- The atomic radii increase with increase in atomic number. This is due to increase in energy levels.
- The group II elements have more protons than group I elements hence this increases the nuclear attraction for the outer electrons.
-
- Cu2+ or copper ions
- Cl- and OH-
-
- Copper pyrites chalcocite, malachite
- To concentrate the ore
-
- Brass
- Batteries
-
- 100 - 25 = 75 cm3
- CxHy + O2 → CO2 + H2O
15 cm3 75 cm3 45 cm3
1 5 3
CxHy + 5 O2 → 3 CO2 + 4 H2O
x = 3 H = 8
C3H8
- Ca(NO3)2 → Ca2+ + 2NO3-
RMM of Ca(NO)2 = 164 onc
Concentration of Ca(NO3)2 = 4.1 g/l
Molarity = Conc. in g/l
RMM
= 0.025M
1 mole Ca(NO3)2 /2 moles Nitrate
0.025 m / 2 x 0.025
0.05M - It would remain unchanged
There is no water to form hypochlorous acid - When aqueous sodium chloride is added to Ca2+. There is no ppt while a white ppt is formed when aqueous sodium chloride is added to a solution containing Pb2+.
-
- N. being a weak acid provides few H+ to be neutralised by OH- hence there is a slight increase in temperature.
- CH3COOH(aq) + KOH(aq) → CH3COOK(aq) + H2O(l)
-
- Experiments 1 and 3.
- In experiment 1, the ions in K2CO3 are tightly held in position and cannot move
while sugar solution does not have ions that can carry a current in solution.
- 1
1 H mass 18
2
1 H mass 20
Download Kenya Certificate Of Secondary Education(KCSE 2013) Chemistry Paper 1 with Marking Scheme.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students