QUESTIONS
SECTION A: BIOLOGY (34 marks)
Answer all the questions in this section in the spaces provided.
-
- State one precaution that should be observed to protect bench tops when performing laboratory experiments. (1 mark)
- State the importance of transport in plants. (3 marks)
- The diagram below represents a terrestrial plant.
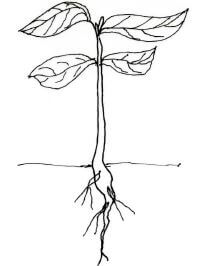
Based on the observation of the diagram:- state the class to which the plant belongs: (1 mark)
- give a reason for your answer in 2 (a) above(1 mark)
- A strip of raw pawpaw measuring 3 cm in length was immersed in a beaker of water. After 30 minutes the strip measured 3.2 cm.
- Explain the change in length.(2 marks)
- State one other observation that was made on the raw pawpaw strip at the end of the experiment.(1 mark)
- The diagram below represents a plant cell as seen under high power of the light microscope.
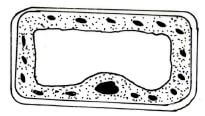
- Based on observations of the diagram, give three reasons why it is a plant cell(3 marks)
- Name the organelle that carries out autotrophic nutrition in plants.(1 mark)
-
- Give a reason in each case why enzymes are:
- catalysts;(1 mark)
- denatured by high temperatures.(1 mark)
- Explain the effect of temperature on the rate of photosynthesis.(2 marks)
- Give a reason in each case why enzymes are:
-
- State two reasons for carrying out vaccination.(2 marks)
- What is an allergic reaction?(1 mark)
-
- Explain how age affects the rate of breathing in human beings.(2 marks)
- Name the causative agent of pulmonary tuberculosis.(1 mark)
-
- The diagrams below represents an experimental set-up that was used to investigate the process of respiration.
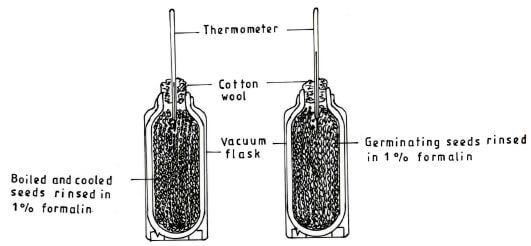
- State the aim of the investigation.(1 mark)
- State the purpose of rinsing the seeds in 1% formalin.(1 mark)
- Name two end products of digestion of a meal consisting of boiled rice and beans without oil.(2 marks)
- The diagrams below represents an experimental set-up that was used to investigate the process of respiration.
-
- Apart from thermoregulation, state two other roles of the skin in homeostasis(2 marks)
- How does amoeba maintain osmotic pressure when placed in hypotonic solution?(2 marks)
- How is the liver involved in thermoregulation?(3 marks)
SECTION B: CHEMISTRY (33 Marks)
Answer all the questions in this section in the spaces provided.
- An experiment was set up to investigate the products of a burning candle as shown in the diagram below.
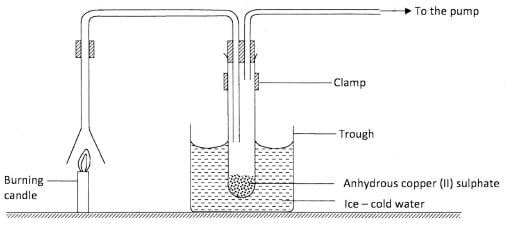
-
- State an observation made on the anhydrous copper (II) sulphate.(1 mark)
- Explain the observation made in a(i) above.(1 mark)
- What effect does the other product of burning candle have on the environment?(1 mark)
-
- The diagram below is a set-up for the laboratory preparation of oxygen gas.
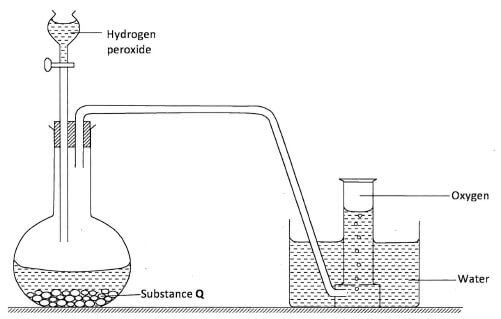
- Name substance Q(1 mark)
- Write an equation for the reaction that produces oxygen gas.(1 mark)
- Name two other reagents which can be used to prepare oxygen in the laboratory.(1 mark)
- Describe a laboratory test to show that the gas produced is oxygen.(1 mark)
- The table below shows the pH values of solutions.
solution M N O P pH 2 7 12 14 - Which solution is likely to be calcium hydroxide?(1 mark)
- Name the products formed when solution M reacts with sodium carbonate.(1 mark)
-
-
- What is meant by the term "hygroscopic salt"?(1 mark)
- Give one example of an hygroscopic salt.(1 mark)
- State one use of sodium chloride other than being a food additive.(1 mark)
-
- A student set up an experiment to investigate the effect of passing an electric current through lead (11) bromide using graphite electrodes as shown below.
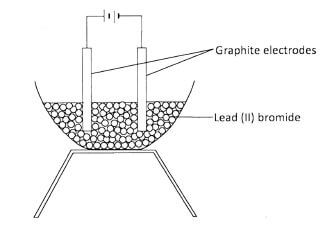
- Identify a mistake in the set-up.(1 mark)
- Name the product formed at the anode after the mistake was corrected.(1 mark)
-
- Hard water is healthy for drinking. Explain.(1 mark)
- Explain how ion-exchange is used to remove hardness in water.(2 marks)
- A student used paper chromatography to separate pigments in different inks and obtained the results shown in the diagram below.
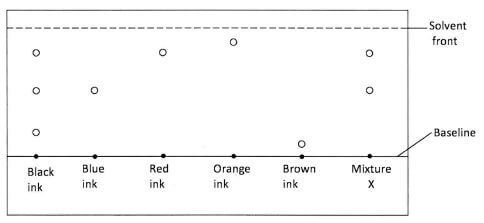
- Identify the inks that are in X.(1 mark)
- Which of the inks is least soluble?(1 mark)
- Describe how sodium chloride can be obtained from a mixture of sulphur powder and sodium chloride.(3 marks)
-
- What is meant by the term "ionization energy"?(1 mark)
- Study the following table and use it to answer the questions that follow. The letters do not represent the actual symbols of the elements.
Element P Q R S T U V W Electronic configuration 2.8.1 2.8.2 2.8.3 2.8.4 2.8.5 2.8.6 2.8.7 2.8.8 1st ionization energy (kJ mol-1) 494 736 576 787 1017 1000 1255 1519 - From the table, ionization energies increase generally from element to element W. Explain.(2 marks)
- What types of oxides are formed by elements:
- R........................................(1 mark)
- U........................................(1 mark)
- The table below shows the atomic numbers of elements of the periodic table represented by letters A to H. The letters are not the actual symbols of the elements.
Elements A B C D E F G H Atomic number 3 7 8 9 10 11 12 13 - Select from the table two elements that belong to the same group.(1 mark)
- Write the formula of a compound formed between elements H and C.(1 mark)
- Which of the elements is monoatomic?(1 mark)
- Select an element that forms a divalent cation(1 mark)
-
- Identify the types of bonds in carbon (II) oxide molecule.(1 mark)
- Explain why graphite is used as a lubricant.(2 marks)
SECTION C: PHYSICS (33 marks)
Answer all the questions in this Section in the spaces provided.
- A stopwatch showed a reading of 0.05 s before the start button was pressed. When this stopwatch was used to measure the time taken by a stone to drop from the top of a building to the ground it recorded 3.68 s. Determine the time of fall.(2 marks)
- Define the term density.(1 mark)
- An object is found to have a weight of 12.5 N at a place where the acceleration due to gravity is 10-2ms. Determine the mass of the object.(3 marks)
- Explain how a drinking straw is used to draw a liquid from a bottle into the mouth.(3 marks)
- State the reason why it is possible to compress a gas but not a liquid.(1 mark)
-
- Overhead power cables between posts are allowed to sag a bit. Explain why this is necessary(2 marks)
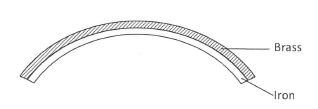
- Figure 1 shows a bimetallic strip made of brass and iron.
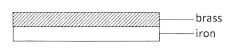
Figure 1
In the space below, draw the appearance of the strip when heated (brass expands more than iron).(1 mark)
- Define the term heat energy.(2 marks)
- Figure 2 shows a uniform metre rule pivoted at the 20 cm mark and balanced by a weight of 4.5 N.

Figure 2
Determine the weight of the rule.(3 marks) - Figure 3 shows a cone resting on a flat surface.
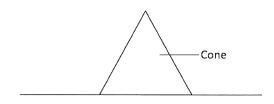
Figure 3- Name the state of equilibrium of the cone.(1 mark)
- State the reason for the answer in (a) above.(1 mark)
- Figure 4 shows a velocity - time graph for the motion of a certain body.
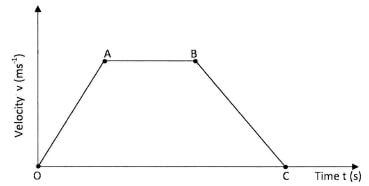
Describe the motion of the body in the region:- OA;(1 mark)
- AB;(1 mark)
- BC.(1 mark)
- The length of a spring is 20 cm. When it is used to support a mass of 0.4 kg, its new length is 20.8 cm. Determine the spring constant. (take acceleration due to gravity g = 10 ms-2)(3 marks)
- It is observed that a passenger standing in a stationary truck tends to fall in a direction opposite to the motion of the truck when it suddenly starts moving. Explain this observation.(2 marks)
- Figure 5 shows a cork floating in water and held in position by a thin thread attached to the bottom of the container
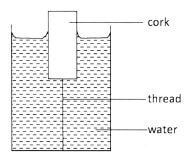
Figure 5
Name three forces acting on the cork.(3 marks) - State the energy changes that take place when a torch is switched on starting with the chemical energy in the cells.(2 marks)
MARKING SCHEME
-
- Avoid spilling chemicals / acids which corrode bench tops;
Avoid cutting specimens on bench tops because they scratch bench surfaces;
Avoid placing hot objects on bench tops which may burn the tops. (1 mark) - Distribution of nutrients / transport of nutrients only;
Availability of raw materials for photosynthesis;
Cooling effect to the plant;(3 marks)
- Avoid spilling chemicals / acids which corrode bench tops;
-
- Dicotyledonae; (1 mark)
- Leaves have net venation;
Have tap root system;(1 mark)
-
- There was movement of water molecules from the beaker into the strip of pawpaw by osmosis;
because the strip tissue fluid was hypertonic / water in the beaker was hypotonic; (2 marks) - The strip became rigid / hard / stiff / turgid / firm;
The strip bent / curved;(1 mark)
- There was movement of water molecules from the beaker into the strip of pawpaw by osmosis;
-
- Peripheral nucleus; Large central vacuole;
Cell wall present; Has regular shape ; /chloroplast present(3 marks) - Chloroplast; (1 mark)
- Peripheral nucleus; Large central vacuole;
-
-
- Control reaction rates; (1 mark)
- They are protein in nature; (1 mark)
- In low temperature the rate of photosynthesis is slow / rate increased with increase in temperature upto to optimum; beyond optimum temperature, rate decreases and eventually photosynthesis stops;(2 marks)
-
-
- To prevent the spread of immunizable diseases;
Enables the individual to develop immunity/to boost/increase immune system; against immunizable infections;(2 marks) - Unusual response by the body cells to a foreign substance; (1 mark)
- To prevent the spread of immunizable diseases;
-
- Metabolism is slower in older people; because of low oxygen demand; leading to lower rate of breathing. (vice versa)(2 marks)
- Micobacterium tuberculosis; (1 mark)
-
-
- To find out if heat if produced by germinating seeds; as they respire. (1 mark)
- To kill bacteria that would respire producing heat;(1 mark)
- Glucose; Amino acids; (2 marks)
-
-
- Excretion of excess salts / water / waste products;
Cooling of the body; by evaporating sweat.(2 marks) - Excess water collects in contractile vacuoles;
which discharge the contents to the exterior;(2 marks)
- Excretion of excess salts / water / waste products;
- Liver has many metabolic activities which release heat; which is
distributed by blood to maintain body temperature;
When it is cold the liver metabolic activities increase /
when it is hot the liver metabolic activities decrease;(3 marks) -
-
- - White 1/2 anydrous copper (II) Sulphate turns blue 1/2 (1 mark)
- - Anhydrous copper (II) Sulphate combines with the water produced to form blue hydrated copper (II) Sulphate. : anhydrous Copper (II) Sulphate becomes hydrated.(1 mark)
- It causes global warming / greenhouse effect. : (1 mark)
-
-
- Q - Manganese (IV) oxide (MnO2). : (1 mark)
MnO2(s) - 2H2O2(aq) → 2H2O(l) + O2(g) : (1 mark)
catalyst - Sodium peroxide and water
OR
Na2O2 and H2O (1 mark)
Penalise 1/2mark for absence or wrong symbols - Oxygen relights / rekindles a glowing splint. :(1 mark)
- Q - Manganese (IV) oxide (MnO2). : (1 mark)
-
- Solution O; (1 mark)
- Salt / sodium salt, carbon (IV) oxide and water :(1 mark)
1 mark - 3 correct
1/2 mark - 2 correct
0 mark - 1 correct
-
-
- Hygroscopic salt refers to a salt that absorbs water from the atmosphere but does not form a solution. : (1 mark)
- KNO3, anhydrous copper (II) sulphate (CuSO4)/anhydrous magnesium chloride and anhydrous calcium chloride. :( ) 1 (1 mark)
- - Manufacture of chlorine
- Manufacture of Sodium carbonate :
- Extraction of sodium metal(1 mark)
-
-
- Absence of source of heat :/ solid PbBr2 instead of molten lead (II) bromide.(1 mark)
- Bromine gas / fumes : (1 mark)
-
- - It provides calcium and magnesium ions 1/2 used by animals in formation 1/2 of strong bones, teeth and shells. (1 mark)
- Hard water is passed through a column packed with a compound of sodium permuttit which exchanges Ca2+ and Mg2+ ions for Na+ ions. (2 marks)
-
- Red and blue (1 mark)
- Brown ink (1 mark)
- - Add water to the mixture, sodium chloride dissolves
- Filter to obtain sulphur
- Evaporate filtrate to obtain sodium chloride. (3 marks) -
- Ionization energy is the minimum energy required to completely remove the outermost electron from an atom in the gaseous state. (w.t.t.e) (1 mark)
-
- The atomic size decrease from P to W because of increase in the effective nuclear charge hence the removal of the outermost electron across the period requires more energy as the atomic size decreases. (2 marks)
-
- R - Amphoteric oxide 1/2
- U - Acidic oxide 1/2 (1 mark)
-
- A 1/2 and F 1/2 (1 mark)
- H2C3 OR Al2O3 (1 mark)
- E (1 mark)
- G (1 mark)
-
- - Covalent bonds1/2
- Coordinate / dative bond 1/2
(1/2 mark for the structure only) (1 mark) - Graphite exist in hexagonal layers which are held together by weak van der waals forces of attraction These layers slip/slide over each other when compressed hence this makes it a good lubricant. (2 marks)
- - Covalent bonds1/2
- Time of fall = 3.68 - 0.05 ; 1
= 3.63s ; 1 - Mass per unit volume / density mass/volume / d = m/v / δ m/v
- W = mg ; 1
12.5 = m x 10 ; 1
m = 1.25 kg ; 1 - On sucking at the open end of the straw, the air inside is removed/sucked out; 1
This lowers/reduces/decreases the pressure inside the straw; 1
The atmospheric pressure now being higher than the pressure inside the straw; 1
forces the liquid up the straw. - The intermolecular distances in gases are larger/greater/higher/larger/more than/bigger than in liquids; 1
-
- This is to allow for contraction ; during cold weather, 1 If this is not done the wires would break ; causing alot of damage. 1
-
- Heat is energy transferred from place to place; 1 as a result of temperature difference; 1
Form of energy; when absorbed/taken in, temperature rises; or when lost/given out, temperature f--- - Sum of anticlockwise moments = Sum of clockwise moments/F1d1 = F2d2
Let weight of rule be x ; 1
x x 30 = 20 x 4.5 ; 1
x = 20 x 4.5
30
= 3 N ; 1 -
- Stable ; 1
- If the cone is slightly displaced, the position of the centre of gravity rises and falls /broad base/larger base/bigger base; 1 back to he same position when the displacing force is removed/vertical line passing through the c.o.g. falls within the base.
-
- The body undergoes uniform/steady/constant acceleration /velocity increasing at uniform rate; 1
- The body moves with constant/steady/uniform velocity; 1 zero acceleration.
- The body moves with a uniform/steady/constant deceleration / velocity decreasing at uniform rate; 1 uniform negative acceleration.
- Extension = 20.8 - 20 = 0.8 cm ; 1
F = Ke
400 x 10 = K x 0 8
1000 100
k = 4 x 100 = 500 Nm-1
0.8 - This is caused by the tendency of the body to remain stationery (inertia); 1 since the body was initially at rest, it tends ; 1 to remain at rest as the truck moves forward.
- - Weight/mg/gravitational
- Upthrust
- Tension - Chemical to electrical
Electrical to light / heat
Download KCSE 2014 General Science Paper 1 Questions with Marking Scheme.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

