QUESTIONS
SECTION A: BIOLOGY (34 marks)
Answer ALL the questions in this section in the spaces provided.
- Differentiate between ecology and ecosystem.(2 marks)
-
- Name three air pollutants produced when charcoal is burnt in a poorly ventilated room.(3 marks)
- Name the causative agent of amoebic dysentery.(1 mark)
- The diagram below represents the human male reproductive system.
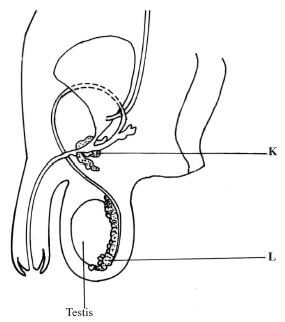
-
- Name the parts labelled K and L.
K: (1 mark)
L: (1 mark) - State the role of the hormone produced by the testis.(1 mark)
- Name the parts labelled K and L.
- What is meant by the term mitosis?(1 mark)
-
-
- What is gestation period?(1 mark)
- State two symptoms of Herpes simplex.(2 marks)
- What is a genotype?(1 mark)
-
- State the meaning of seed viability.(1 mark)
- State two reasons why water is required for seed germination.(2 marks)
-
- Giving an example, describe continuous growth in animals.(2 marks)
- Distinguish between the terms homozygosity and heterozygosity.(2 marks)
-
- What is chemical evolution?(2 marks)
- State two ways in which meiosis is important in sexual reproduction.(2 marks)
- State the meaning of the following terms:(3 marks)
- irritability:
- stimulus;
- response.
- Name three structures of the human ear that are involved in balance and posture.(3 marks)
- State three functions of an endoskeleton.(3 marks)
SECTION B: CHEMISTRY (33 marks)
Answer ALL the questions in this section in the spaces provided.
- The set-up shown below was used to investigate some properties of chlorine gas.
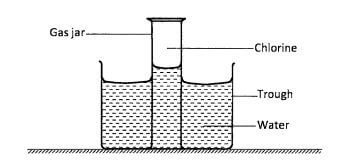
- Explain why the level of water in the gas jar was higher than in the trough after some time.(1 mark)
-
- What would be observed if blue litmus paper was dipped into the water in the trough?(1 mark)
- Explain the observations made in b(i) above.(2 marks)
- Calculate the number of moles contained in 30g of potassium nitrate (K = 39.0; N= 14.0; 0 = 16.0).(2 marks)
- A balloon filled with air was tied and held above a trough containing hot water as shown in the diagram.
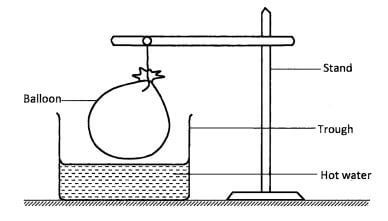
- State the observation made on the balloon.(1 mark)
- Explain the observation in (a) above.(2 marks)
-
- What is meant by the term dilution?(1 mark)
- Calculate the mass in grams contained in 25.0 cm3 of 0.2M sodium hydroxide solution (Na = 23.0; O = 16.0; H = 1.0).(2 marks)
-
- Name one natural polymer and state its use.(1 mark)
Natural polymer.
Use. - State one advantage and one disadvantage of synthetic polymers.(1 mark)
Advantage........................................................................................................
Disadvantage........................................................................................................
- Name one natural polymer and state its use.(1 mark)
-
- Iron metal exists naturally in different ores. Other than haematite, name another common ore of iron.(1 mark)
- During the extraction of iron metal, one of the reactions in the blast furnace is:
Fe2O3(s) + 3CO(g) → 2Fe(l) + 3CO2(g)- Name the raw material that is used to produce carbon (II) oxide.(1 mark)
- Iron metal produced in the reaction is in liquid state. Explain.(1 mark)
- State with a reason, one use of stainless steel.(2 marks)
Use:........................................................................................................
Reason:........................................................................................................
- The set-up shown below was used by a student to prepare sulphur (IV) oxide gas. Study it and answer the questions that follow.
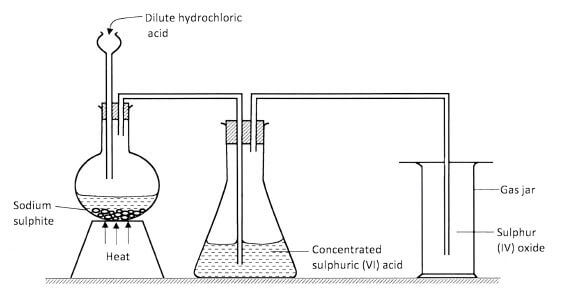
-
- Identify a mistake on the set-up that will affect collection of sulphur (IV) oxide gas.(1/2 mark)
- How would the mistake be corrected?(1/2 mark)
-
- State the use of concentrated sulphuric (VI) acid in the above set-up.(1 mark)
- What would happen if concentrated sulphuric (VI) acid was replaced with water?(1 mark)
- State one use of sulphur (IV) oxide gas.(1 mark)
-
- When Potassium chloride was dissolved in water, the following change occurred.
KCl(s) + H2O(l) → KCI(aq) ; ΔH = + 4.97 kJmol--
- State the type of energy change in the above reaction.(1 mark)
- The above experiment was done in a boiling tube. State the observation that was made.(1 mark)
- Name the type of reaction in a(ii) above.(1 mark)
- Name two factors considered when choosing a fuel.(2 marks)
-
-
- Name the compound CH3CHCHCH3 (1 mark)
- Name the type of reaction that takes place when the compound in (a) above is reacted with hydrogen chloride gas.(1 mark)
- 0.1 M hydrochloric acid was reacted with sodium thiosulphate solution. The time taken for the cross to disappear was recorded at different temperatures as shown on the graph.
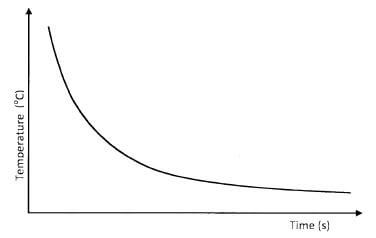
- Explain the shape of the curve.(1 mark)
- What conclusion would be made from the curve?(1 mark)
- Sketch another curve on the same axis that would be obtained when the concentration of hydrochloric acid is doubled.(1 mark)
SECTION C: PHYSICS (33 marks)
Answer ALL the questions in this section in the spaces provided.
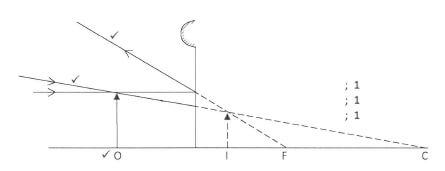
- Figure 1, shows an image I formed when an object O is placed infront of a convex mirror.
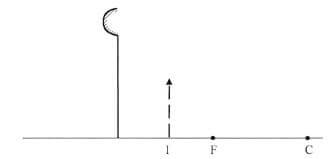
Figure 1
Complete the ray diagram to show the position of object O.(3 marks) - When a polythene rod is rubbed with a dry piece of cloth, and then brought near a negatively charged pith ball, the ball is observed to move away. Explain this observation.(2 marks)
-
- Name one defect of a simple cell.(1 mark)
- State how the defect in (a) above is minimized. (1 mark)
- Figure 2 shows iron keepers used in storing bar magnets.
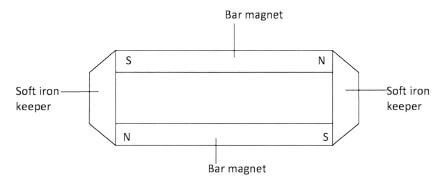
Figure 2
On the figure show the poles induced in the keepers.(1 mark) - Figure 3, shows an arrow which indicates the direction of travel of a wave in a medium. P, a particle of the medium is in the path of the wave.

Figure 3
In the space provided, sketch the diagram to show how the particle P moves when the wave is- transverse.
- longitudinal(2 marks)
- State two factors that affect the speed of sound in air.(2 marks)
- Define the term potential difference.(1 mark)
- Figure 4, shows two circuits X and Y in which two identical coils are used to heat two equal amounts of water. The two circuits are switched on at the same time.
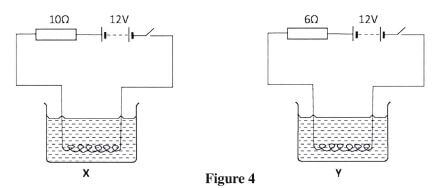
Figure 4- State the circuit in which the water boils first.(1 mark)
- Explain the answer in (a) above.(2 marks)
- It is observed that a swimming pool full of water appears shallower than it actually is. Explain this observation.(3 marks)
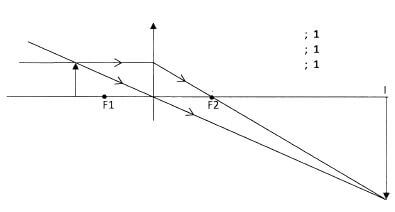
- Figure 5, shows an object O placed in front of a converging lens whose principal foci are F1 and F2.
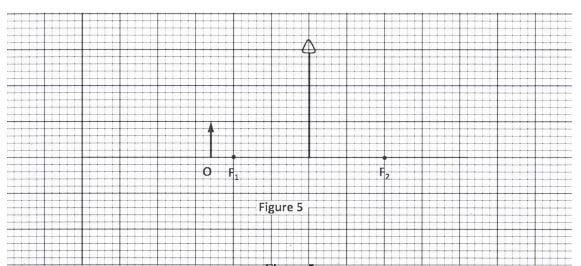
Figure 5
Using rays, complete the diagram to show the position of the image.(3 marks) - Figure 6, shows a displacement - time graph of a wave.
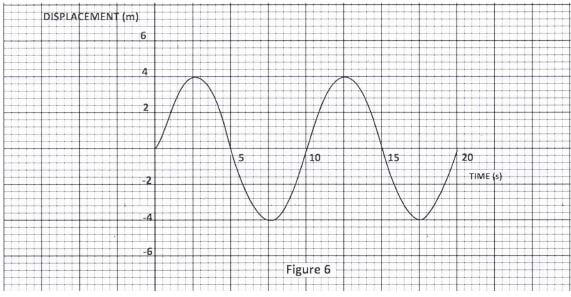
Figure 6
Determine the amplitude of the waveform.(1 mark) - An electric iron is rated 1500 W. Determine the cost of using the iron for 30 hours given that the cost of electricity is Ksh.8 per kilowatt hour.(3 marks)
-
- State one way in which the path of a cathode ray can be changed.(1 mark)
- The control grid in a cathode ray oscilloscope (CRO) is used to control the brightness of the spot on the screen. Explain how the brightness of the spot may be reduced.(2 marks)
- State two ways in which the conductivity of a semiconductor can be increased.(2 marks)
- Explain the danger of radioactive emissions on a human body.(2 marks)
MARKING SCHEME
- Ecology is the study of interaction of organisms with one another and with their physical environment while Ecosystem is the sum total of the interactions linking organisms in a community with one another and with their environment.(2 marks)
-
- Carbon (II) Oxide;
Carbon (IV) Oxide;
Carbon particles / soot;(3 marks) - Entamoeba histolytica;(1 mark)
- Carbon (II) Oxide;
-
-
- K - Prostate gland; L - Epididymis;(2 marks)
- Controls development of secondary sexual characteristics; leads to formation of spermatozoa/sperms;(1 mark)
- It is a type of cell division giving rise to two identical diploid daughter cells; (1 mark)
-
-
- The time between implantation and birth.(1 mark)
- Genital sores /painless sores;
Ulcers in affected areas;(2 marks) - It is the genetic constitution / make up of an organisms;(1 mark)
-
- Ability of a seed to germinate; (1 mark)
- It softens the seed coat;
It hydrolyses stored food;
It is a medium for enzyme activity / activates enzymes / transports dissolved nutrients; (2 marks)
-
- In continuous growth, the animal grows all the way from fertilization till maturity; e.g. height in humans;(2 marks)
- Homozygosity is a condition in which the gene pairs are similar / same / identical while Heterozygosity is a condition in which the gene pairs are different. (2 marks)
-
- It is a theory on the origin of life; that suggests that life began from simple elements through complex compounds;(2 marks)
- Meiosis leads to the formation of the gametes which are haploid;
Meiosis ensures that the chromosomal constitution of offspring is the same as that of parents;(2 marks)
-
- Irritability - ability to detect and respond to changes in the environment; (1 mark)
- Stimulus - A detectable change in the environment capable of producing a response; (1 mark)
- Response - the change in activity by the organism due to a stimulus.(1 mark)
- Semicircular canals;
Utriculus;
Sacculus;(3 marks) - Give the body its shape;
Protects delicate internal organs;
Provides surface for attachment of muscles;
Produces / manufacture blood cells;
Stores calcium and phosphate ions;
Enables/allows movement;
Gives body balance and posture;
Supports the body wight. (3 marks) -
- Chlorine gas is slightly soluble in water hence water rises to occupy the space which was occupied by the dissolved gas. (1 mark)
-
- Turns red then bleached
OR
decolourised / turned white (1 mark) - Chlorine gas dissolves in water to form acidic solution which turns blue to red.
The red litmus paper is further decolourised / bleached due to presence of hypochlorous acid or chloric (I) acid.(2 marks)
- Turns red then bleached
- Formula mass of KNO3= 39 + 14 + 3 (16)
= 53 + 48
= 101 g
Number of moles in 30 g = 30/101
= 0.2970 moles (2 marks) -
- The balloon increased / expanded in size/volume.(1 mark)
- Rise in temperature increases kinetic energy of air molecules in the balloon hence increased collisions / bombardments against the walls of the balloon, causing the balloon to expand. (2 marks)
-
- A process where a solvent is increased/water is added/increased while the amount of solute remains constant in a given solution. (1 mark)
- Formula mass of NaOH = 23 + 16 + 1 = 40 g
Moles in 25cm3 = 0.2 x 25
1000
Mass in 25cm3 of NaOH = 0.2 x 25 x 40
1000
= 0.2 g (2 marks)
-
- Natural polymers
Cellulose / proteins / natural rubber/silk/wool.
Uses
Used for paper manufacturing, textiles
- Rubber used in tyres, tubes
- Proteins / cellulose in textiles.(1 mark) -
- Advantages of synthetic polymer
- Cheap / long lasting / moulded into many shapes.
- Prevent / safe destruction of plants and animals.
- Some are heat resistant / good insulators / non corrosive to acids / alkalis. (any one correct for 1/2) - Disadvantage of synthetic polymer
- Pollutants to the environment
- Non-biodegradable
- Costly to recycle
- Burn producing poisonous gases. (1 mark) (any one correct at 1/2 mark) 2 marks
- Advantages of synthetic polymer
- Natural polymers
-
- Magnetite / Pyrite.
OR
FeCO3 / FeS (1 mark) -
- Coke /Carbon (1 mark)
- Temperatures at the blast furnace are higher than the melting point of iron metal. (1 mark)
- Use of stainless steel
- Construction of bridges
- In ships
- Pipes, padlocks
- Nails
- Cutlery.(1 mark)
(any one correct for 1 mark)
Reason: Stainless steel does not rust /is resistant to corrosion. (1 mark)
- Magnetite / Pyrite.
-
-
- The thistle funnel is above the reagents and allows the gas to escape
OR
The thistle funnel doesn't have a tap. ( 1/2 mark) - Use a dropping funnel / Dip the thistle funnel into the reagents. ( 1/2 mark)
- The thistle funnel is above the reagents and allows the gas to escape
-
- Used to dry the sulphur (IV) oxide/as a drying agent.
- The sulphur (IV) oxide gas would dissolve in the water, hence no gas would be collected in the gas jar.
- Uses of sulphur (IV) oxide
- Manufacture of sulphuric (VI) acid / sulphuric acid/ H2SO4 (l)
- Bleaching agent
- As a refrigerant
- Fruit preservative
- Infumigation/to kill germs (1 mark)
(any one correct for 1 mark)
-
-
-
- Heat / enthalpy of solution (1 mark)
- The temperature would drop / boiling tube becomes cold. (1 mark)
- Endothermic reaction. (1 mark)
- - Heat value
- Cost of fuel
- Availability
- Environmentally friendly/less pollution
- Cost of transporting the fuel/toxicity of fuel (2 marks)
(Any two correct for 2 marks)
-
-
- But - 2 - ene (1 mark)
- Addition reaction (1 mark)
-
- As the temperature rises, the time taken for the cross to disappear decreases.
OR
as the temperature decreases, the time taken for the cross to disappear increases. (1 mark) - The rate of reaction increases with rise in temperature.
OR
The rate of reaction decreases with decrease in temperature. (1 mark) - Diagram.
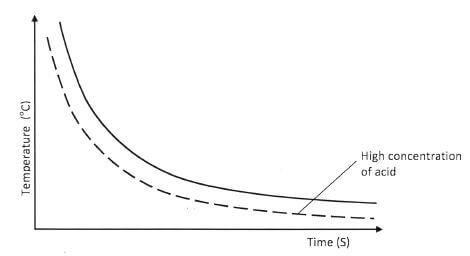
(1 mark)
- As the temperature rises, the time taken for the cross to disappear decreases.
-
- When the polythene rod is rubbed it acquires a negative charge; On bringing it near. 1 a negatively charged pith ball the two repel one another; 1
-
- Polarization / local action/polarization/accummulation of hydrogen bubbles around the cathode 1
- Polarization is minimised by adding depolarizer /pottasium dichromate/ local action/ dissolving of zinc/zinc being eaten / local action is minimised by coating zinc with mercury/amalgamation/using pure zinc. 1
-

-
-
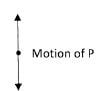
Perpendicular oscilation. -

Parallel along direction of the wave.
-
- - Temperature; 1
- Humidity; 1
- Density of air - Work done in moving a unit charge from one point to another; 1
-
- Circuit Y; 1
- More current is flowing due to less; 1
resistance in the circuit hence a greater heating effect; 1
- - Light rays from the bottom of the pool are refracted at the interface; 1
- The ray of light from the bottom is bend away from the normal; 1
- The image of the bottom appears raised; 1 -
- a = 4 m; 1
- 1500 ÷ 1000 = 1.5 kW ; 1
Cost of electricity = 1.5 x 30 x 8; 1
= Ksh.360 ; 1 -
- By using a magnetic or electric field ; 1
- - By increasing the grid potential so that/making the grid more negative; 1 fewer electrons reach the screen/more electrons are repelled; 1 34.
- - By raising the temperature of the semiconductor; 1
- By doping the semiconductor ; 1 - - Radiations have high energy ; 1 which damages the body cells; 1
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download KCSE 2014 General Science Paper 2 Questions with Marking Scheme.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

