General Science Paper 1 (237/1)
SECTION A: BIOLOGY (34 marks)
Answer all the questions in this section in the spaces provided.
- Name a branch of Biology that deals with the study of animals.(1 mark)
- State two domestic applications of anaerobic respiration.(2 marks)
-
- Define the following terms as used in cell physiology:
- diffusion (1 mark)
- active transport (1 mark)
- State how support is achieved in young herbaceous plants.(1 mark)
- Define the following terms as used in cell physiology:
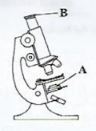
- The diagram below represents a light microscope.
- Name the part labelled B.(1 mark)
- State the function of the part labelled A.(1 mark)
- Identify two organelles of an animal cell that would be seen under the light microscope.(2 marks)
-
- What is the meaning of the term excretion?(1 mark)
- State two reasons why excretion is necessary in animals.(2 marks)
- The scientific name of a lion is Panthera Leo. Classify the organism under the following taxonomic units:
- class(1 mark)
- genus(1 mark)
- Describe absorption of water from the soil by the root hairs.(3 marks)
-
- Name two members of the Kingdom Protoctista.(2 marks)
- Name two causes of liver cirrhosis.(2 marks)
- Explain the significance of the following in the feeding of a mammal
- long tongue in herbivores (2 marks)
- canines in carnivores (1 mark)
-
- Explain the importance of gaseous exchange in humans.(2 marks)
- Name the main gaseous exchange structure in terrestrial plants.(1 mark)
-
- State the role of enzymes in metabolic processes.(1 mark)
- Explain the difference in energy requirements for a man and a woman of the same age.(2 marks)
-
- Explain why ventricles have thicker walls than the auricles.(2 marks)
- State the role of platelets in the human body.(1 mark)
SECTION B: CHEMISTRY (33 marks)
Answer all the questions in this section in the spaces provided.
- Table 1 shows the pH values of various solutions. Use it to answer the questions that follow.
Table 1
Solution F E D H G pH 1.0 7.0 12.0 6.5 10.5 - Identify the nature of the substance formed when F and D react.
- Identify the solution likely to be a:
- weak acid
- weak base
-
- A student prepared an insoluble salt by mixing two different salt solutions.
- Identify the method used to prepare the insoluble salt.(1 mark)
- Name one other method which can be used to prepare insoluble salts.(1 mark)
- Give one industrial use of sodium carbonate salt.(1 mark)
- A student prepared an insoluble salt by mixing two different salt solutions.
-
- A student used salt solution to remove blood stains from the school uniform. Name the method of separation the student applied.(1 mark)
- Anhydrous calcium chloride when left in the open forms a solution.
- Give the term used to describe such a substance.(1 mark)
- State one major application of such a substance.(1 mark)
- A sample of hard water was divided into three portions. Table 2 shows the tests and observations made on each portion.
Table 2
Portion Test Observation 1 1cm³ of soap added and shaken. No lather was formed. 2 Boiled and cooled. 1cm³ of soap was added and shaken. No lather was formed. 3 3cm³ of sodium carbonate was added and filtration done. 1cm³ of soap was added to the filtrate and shaken. Lather formed immediately. - Name the type of water hardness that was present in the sample.(1 mark)
- Identify two anions present in the water sample.(1 mark)
- Give one other substance that can be used for portion three instead of sodium carbonate.(1 mark)
-
- Distinguish between a covalent bond and a co-ordinate bond. (2 marks)
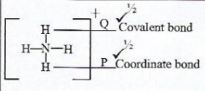
- Figure 1 is a diagram of ammonium ion.
Name the type of bond labelled. (½ mark)- P
- Q (2 mark)
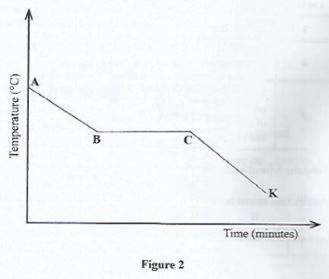
- Figure 2 represents a cooling curve for a liquid. Study it and answer the questions that follow.
- Explain why the temperature remained constant at region B and C.(2 marks)
- Give the physical state of the substance at region CK.(1 mark)
-
- Name the chemical family of the elements Helium, Neon and Aryon.(1 mark)
- Elements V, X, Y and Z belong to the same group in the periodic table. Table 3 gives information about the elements. Use it to answer the questions that follow. The letters do not represent the actual symbol of the elements.
Table 3
Explain the trend in:Element Atomic radi (am) Melting point (°C) V 0.152 180 X 0.186 98 Y 0.231 64 Z 0.244 39 - atomic radii (1 mark)
- melting point (1 mark)
-
- Define the term electrolyte.(1 mark)
- Mercury and molten lead(II) bromide are good conductors of electricity. Explain how each one of them conducts electricity.(2 marks)
-
- Name two subatomic particles.(1 mark)
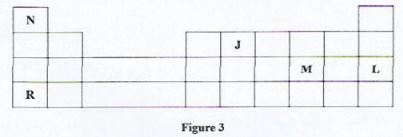
- Figure 3 shows part of a periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbol of the elements.
- State the period to which element J belongs. (1 mark)
- Write the formula of the compound formed when element R reacts with element M.(1 mark)
- State the nature of the oxide formed by element N. (1 mark)
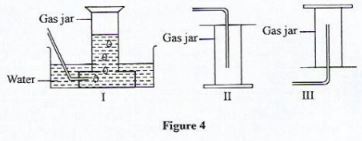
- The set-ups I, II and III in Figure 4 shows different methods of gas collection used in the laboratory. Use it to answer the questions that follow.
- Identify the set-up used to collect dry hydrogen gas.(1 mark)
- State one property of the gas collected using set-up II.(1 mark)
- Name the method of gas collection in set-up I.(1 mark)
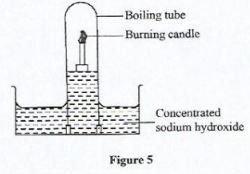
- Figure 5 shows an experimental set-up used to investigate the active part of air. Study it and answer the questions that follow.
- In the space provided next to Figure 5 draw a diagram to show observations made at the end of the experiment.(2 marks)
- Explain the role of concentrated sodium hydroxide in the experiment.(1 mark)
SECTION C: PHYSICS (33 marks)
Answer all the questions in this section in the spaces provided.
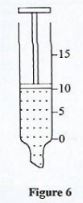
- Figure 6 shows a syringe containing a liquid.
The mass of the syringe in Figure 6 when empty is 20g and when filled with some liquid, it weighs 30 g. Determine the density of the liquid. (the syringe is graduated in cm)(3 marks) - Explain why a drop of water placed on a clean glass surface spreads out.(2 marks)
- A beaker of height 0.15 m is filled with a liquid of density 13600 kgm due to the liquid at the bottom of the beaker. (g = 10 Nkg). Determine the pressure(3 marks)
- A student observed that dust particles illuminated by a beam of light in a room moved in a constant random motion. Explain this observation.(2 marks)
- State the three modes of heat transfer.(3 marks)
- State two reasons why water is not suitable for use as a thermometric liquid.(2 marks)
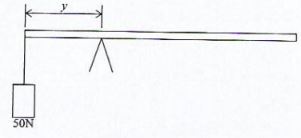
- Figure 7 shows a uniform plank of length 2m and a weight of 75N. It is pivoted at a distance v from one end and balanced by a weight of 50N.
Determine the value of y.(3 marks) - Figure 8 shows a stone resting on a horizontal surface.
Explain the effect on the stability of the stone when the shaded part is chopped off.(2 marks) - A certain mass was attached to a spring. When the mass was removed, it was observed that the Spring did not regain its original length. State the reason for this observation.(1 mark)
- A train moving at a velocity of 25 ms decelerates uniformly and comes to rest in 10 seconds. It then starts moving again after 5 seconds and accelerates uniformly to a velocity of 10 ms in 5 seconds. Sketch a velocity-time graph for the motion of the train within this period.(3 marks)
- A spherical marble rolls freely on a floor until it comes to rest. State two factors that determine the distance it covers before stopping.(2 marks)
- A stone is thrown vertically upwards and returns to the ground after some time.
State the energy changes that take place.(2 marks) - It is observed that a boat sinks more in fresh water than in sea water. Explain this observation,(3 marks)
- State two reasons why it is necessary to tidy up the laboratory after a physics experiment.(2 marks)
MARKING SCHEME
- Zoology: RJ wrong spelling.
-
- Production of fermented dairy products e.g. yoghurt;
- Production of alcoholic beverages;
- Production of leavened bread;
- Fermented porridge:
-
-
- Dillusion process by which particles/molecules move from a region ofhigh concentration to a region of low concentration;
- Active transport - process by which particles move from a region of low concentration to a region of high concentration with the use expenditure of energy:
- By cells becoming turgid/turgidity;
-
-
- Eye piece; Acc eye piccc lens.
- Concentrates/converge/condense light onto the specimen;
-
- Nucleus;
- Cytoplasm;
- Cell membrane;
-
- Elimination of waste product of metabolism from the body of a living organism through an excretory organ;
-
- Removes toxic/harmful substances from the body;
- For osmoregulation; Acc. Water and salt balance
-
- Mammalia;
- Panthera;
- Root hairs are surrounded by a film of water in the soil; the cell sap of the root hairs contains salts and sugars, hence is more concentrated/ hypertonic; Water is drawn into the root hairs by osmosis; across the semi-perineable membrane of the root hair cells;
-
-
- Amoeba;
- Paramecium;
- Euglena;
- Plasmodium.
- Spirogyra;
- Chlamydomonas;
(Any two) RJ wrong spelling. (b)
-
- Excessive intake of alcohol;
- Infection by liver parasites/bacteria/ virus;
-
-
-
- Assist in cutting grass
- Turning/manipulation of grass;
- Piercing/tearing/griping;
-
-
-
- Enables cells/tissues get oxygen for respiration;
- Elimination of carbon (IV) oxide gas;
- Stomata/Stoma;
-
-
- Regulates the rate of metabolic processes (slow/accelerate/speed the rate);
- A man needs more energy than a woman; a man has more muscles/is more muscular, hence needs for more energy for constant muscular contraction/ relaxation; RJ Masculine,
-
- Ventricles pump blood for longer distances; thus need thick muscles to withstand high pressure/generate high pressure.
- Blood clotting/stops bleeding:
-
- Neutral Substance
-
- H
- G
-
-
- Double decomposition/Precipitation
- Direct synthesis
- Softening hard water, manufacture of glass
-
-
- Solvent extraction
-
- Deliquescent
-
- Manufacture of glass
- Making detergents
- As a drying agent
- Manufacture of papers
Any one @ 1 mark
-
- Permanent hardness
- Chloride ions, sulphate ions
- Ion exchange
-
-
- Covalent bond is formed by equal contribution of the shared electrons by the atoms.
- Coordinate bond is forned when shared electrons are contributed by a single species of the atom.
-
-
-
-
- Liquid = Solid phase
- Temperature remain constant as Kinetic energy reduces, articles, form bonds with each other coming closer to form solids. Energy produced is used in bond formation/ substance is changing state
- Solid
-
-
- Noble gases
-
- Atomic Radius Incrcascs downthe group due to increase in the number of energy levels.
- Melting point decreases down the group, the forces of attraction between atoms weakens hence decrease in melting point.
-
- Is a substance when in solution/melt conducts and decomposes by passage of an electric current.
- Mercury contains delocalized electrons (free electrons) which conduct electricity while Lead (II) bromide in molten state contains IONS (Pb2+, Br) which conduct electricity.
-
-
- Electrons
- Protons
- Neutrons
Maximum 1 mark: two correct and above
-
- Period 2
- RM
- Neutral oxide
-
-
- III
- Denser than air
- Over water method
-
-
- To absorb Carbon (IV) oxide produced after combustion.
-
- ρ=m/v
=10/10
1gcm3 - Adhesion between the water molecules and the glass surface is higher v than the cohesion between the water molecules hence the water spreads. OR cohesion is lower than adhesion. P= Phg
- P=ρhg
13600x0.15x10
= 20,400Pa (N/m2) - The dust particles are bombarded/knocked/hit by invisible air✓ molecules which are in constant random motion.
-
- Conduction
- Convection
- Radiation
-
- It wets/sticks on glass
- It has a low range of temperature /high freezing and low boiling point.
- It expands unusually/ doesn't expand uniformly/anomalous expansion
- It is a poor conductor of heat
- Not visible (any 2)
- Sum of clockwise moment=sum of anticlockwise moment
F₁d₁ = f₂d₂
50 x y=75(1- y)
50 y = 75–75
y = 75/125
= 0.6m - The stone becomes less stable since the center of gravity shifts to the left unshaded part
- The mass (force) stretched the spring beyond its elastic limit./elastic limit exceeded/break point
-
-
- Frictional force between marble and floor/Nature of the floor.
- Initial speed of the marble/initial force applied
- Mass/weight of the marble.
- Steepness
- Kinetic energy → potential energy → kinetic energy
→ (sound/heat) - The weight of the water displaced/up thrust in both cases is the same, the less dense (fresh water) ✓ more volume will be displaced.
-
- Ensure proper storage of apparatus. /to locate apparatus easily.
- To minimize risk of accidents/injuries.
- Minimize breakages.
Download KCSE 2019 General Science Paper 1 Questions with Marking Scheme.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students