Chemistry Paper 3 (233/3)
- You are provided with:
- Solution A: aqueous Iron(III) sulphate.
- Solution B: aqueous potassium iodide.
- Solution C: mixture of aqueous starch and sodium thiosulphate solution.
You are required to determine the rate of reaction between aqueous Iron(III) sulphate (solution A) and aqueous potassium iodide (solution B). - Procedure:
- Place 5 test tubes on a test tube rack and label them 1, 2, 3, 4 and 5. Fill a burette with solution A. To each test tube place 3 cm³ of solution A from the burette.
- Clean the burette and fill it with solution B. Place 8 cm³ of solution B into a 100 ml beaker from the burette.
- Using a 10ml measuring cylinder, add 2cm³ of solution to the beaker containing solution B followed by 7 cm³ of distilled water measured using the same 10 ml measuring cylinder.
- Pour the contents of test tube 1 to the mixture in the beaker and immediately start the stop watch. Swirl the contents of the beaker. Record in table 1 the time taken for a blue colour to just appear. Measure the temperature of the final mixture and record in the space provided. Wash the beaker and proceed to step (v).
- Place 6cm³ of solution B into 100 ml beaker from the burette. Add 2cm³ of solution C followed by 9 cm³ of distilled water. Add solution A in test tube 2 to the mixture in the beaker and immediately start the stop watch. Swirl the contents of the beaker. Record in table 1 the time taken for a blue colour to just appear. This is experiment 2.
- Wash the beaker. Repeat step (v) with solution A in test tubes 3, 4 and 5 with corresponding volumes of solution B, solution C and distilled water as indicated in table 1 for experiments 3, 4 and 5.
- Temperature of final mixture .....................°C(1 mark)
- Table 1
(3 marks)Experiment Volume (cm³) of Time
(seconds)Solution
ASolution
BSolution
CDistilled
Water1 3 8 2 7 2 3 6 2 9 3 3 5 2 10 4 3 4 2 11 5 3 3 2 12 - Complete table 2 for each experiment by:
- calculating the square of volume of solution B, BP and filling in the table.
- calculating the rate of reaction which is given by the expression
Rate =1/time X 1000 s-1 and filling in the table.
Table 2
(5 marks)Experiment B2 Rate =1/time X 1000 s-1 1 2 3 4 5
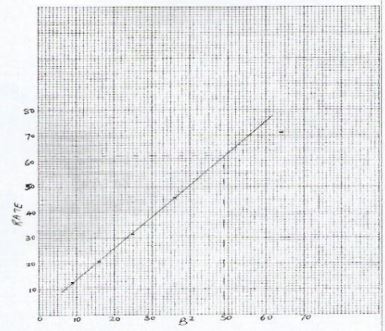
- Plot a graph of rate (y-axis) against B (3 marks)
- Using the graph, determine the time that it will take for the blue colour to appear if the experiment is repeated using the following mixture:
(2 marks)Volume (cm³) of Solution A Solution B Solution C Distilled water 3 7 2 8 - In this experiment the rate of reaction was determined with respect to potassium iodide. Describe how the rate of the reaction can be determined with respect to Iron(III) sulphate. (2 marks)
- You are provided with solid P. Carry out the following tests and record the observations and inferences in the spaces provided.
- Place about one-third of solid P in a dry test tube and heat it strongly. Test any gases produced with red litmus paper.
Observations Inferences (2 marks) (1 mark) - Place the remaining amount of solid P in a boiling tube. Add about 15cm of distilled water and shake to dissolve the solid. Use about 2 cm³ portions of the solution in a test tube for each of the tests (i) to (iv).
- To the first portion of the solution add aqueous sodium hydroxide.
Observations Inferences (1 mark) (2 marks) - To the second portion of the solution add 2 or 3 drops of aqueous barium nitrate.
Observations Inferences (1 mark) (1 mark) - To the third portion of the solution add 2 or 3 drops of aqueous lead(II) nitrate. Warm the mixture.
Observations Inferences (1 mark) (1 mark) - To solid D in the test tube add about 2 cm of distilled water. Shake and label this as chlorine water. Add all the chlorine water to the fourth portion of the solution. Shake the mixture and then add 3 drops of starch solution.
Observations Inferences (2 marks) (1 mark)
- To the first portion of the solution add aqueous sodium hydroxide.
- Give the formulae of the ions present in solid P:
- cation
- Anion
- Place about one-third of solid P in a dry test tube and heat it strongly. Test any gases produced with red litmus paper.
- You are provided with liquid Q. Carry out the following tests and record the observations and inferences in the spaces provided.
- Place 2 drops of liquid Q on a watch glass. Ignite the liquid with a Bunsen bumer flame.
Observations Inferences (1 mark) (1 mark) - Place about 2 cm of liquid Q in a test tube. Add about 2 cm of distilled water and shake the mixture.
Observations Inferences (1 mark) (1 mark) - To about 2 cm of liquid Q in a test tube, add all of the solid sodium hydrogen carbonate provided
Observations Inferences (1 mark) (1 mark) - To about 2 cm of liquid Q in a test tube, add 2 or 3 drops of bromine water.
Observations Inferences (1 mark) (1 mark) - To about 2 cm3 of liquid Q in a test tube, add 2 or 3 drops of acidified potassium dichomate(VI) and warm the mixture.
Observations Inferences (1 mark) (1 mark)
- Place 2 drops of liquid Q on a watch glass. Ignite the liquid with a Bunsen bumer flame.
MARKING SCHEME
-
- Temperature of final mixture 22,5°C (1 mark)
- Table 1
(3 marks)Experiment Time
(seconds)1 14.1 2 22.0 3 32.0 4 49.0 5 78.2
Complete table (1½ mark)
Decimal (½ mark)
Accuracy (½ mark)
Trend (½ mark) - Table 2
Note: All points lie on a straight line except for B=8, B2 = 64, rate = 70.9S-1Experiment B2 Rate =1/time X 1000 s-1 1 64 70.9 2 36 45.5 3 25 31.3 4 16 20.4 5 9 12.8
Correctly worked B2.
each value to a maximum of 2'/2 marks
Correctly worked rate
for each value to a maximum of 22 marks
(5 marks) -
Scale-1/2
Labelled Axes - 1/2
Line - 1
Plots 1
(3 marks) - B=7, B2 -= 49
From the graph, Rate = 1000/time = 62
∴time= 1000/62=16.1s - Keep volume of solution B and solution C constant. Use different volumes of solution A Calculate appropriate volume of distilled water to use to make total volume constant. (2 marks)
- You are provided with solid P. Carry out the following tests and record the observations and inferences in the spaces provided.
- Place about one-third of solid P in a dry test tube and heat it strongly. Test any gases produced with red litmus paper.
Observations Inferences -red litmus paper turns blue
- white fumes which form a white solid on the sides of the test tube.
OR
- the white solid sublimes.an ammonium salt OR basic gas
OR
NH+₄ ions present.(2 marks) (1 mark) - Place the remaining amount of solid P in a boiling tube. Add about 15cm of distilled water and shake to dissolve the solid. Use about 2 cm³ portions of the solution in a test tube for each of the tests (i) to (iv).
- To the first portion of the solution add aqueous sodium hydroxide.
Observations Inferences No white precipitate Inferences Zn2+ /Pb2+ Al3+/Mg2+ / Ca2+ ions absent (1 mark) (2 marks) - To the second portion of the solution add 2 or 3 drops of aqueous barium nitrate.
Observations Inferences No white precipitate Inferences SO2-4/SO2-3/CO2-3 ions absent (1 mark) (1 mark) - To the third portion of the solution add 2 or 3 drops of aqueous lead(II) nitrate. Warm the mixture.
Observations Inferences White precipitate (V2 mark), which dissolves
on warmingCl- or Br- ions present (1 mark) (1 mark) - To solid D in the test tube add about 2 cm of distilled water. Shake and label this as chlorine water. Add all the chlorine water to the fourth portion of the solution. Shake the mixture and then add 3 drops of starch solution.
Observations Inferences Colourless solution turns yellow (1 mark) Yellow colour persists OR no blue colour with starch. Br- ions present
I- ions absent(2 marks) (1 mark)
- To the first portion of the solution add aqueous sodium hydroxide.
- Give the formulae of the ions present in solid P:
- cation - NH+4
- Anion - Br-
- Place about one-third of solid P in a dry test tube and heat it strongly. Test any gases produced with red litmus paper.
- You are provided with liquid Q. Carry out the following tests and record the observations and inferences in the spaces provided.
- Place 2 drops of liquid Q on a watch glass. Ignite the liquid with a Bunsen bumer flame.
Observations Inferences - Burns with a yellow flame Long chain hydrocarbon or unsaturated compound (1 mark) (1 mark) - Place about 2 cm of liquid Q in a test tube. Add about 2 cm of distilled water and shake the mixture.
Observations Inferences Two layers formed
OR
Q not soluble/ immiscible in waterNon-polar compound (1 mark) (1 mark) - To about 2 cm of liquid Q in a test tube, add all of the solid sodium hydrogen carbonate provided
Observations Inferences No effervesence Q is not acidic
OR
Absence od carboxylic acid
OR
RCOOH/H+/H3O+(1 mark) (1 mark) - To about 2 cm of liquid Q in a test tube, add 2 or 3 drops of bromine water.
Observations Inferences Bromine water not decolourised Alkene/alkyne absent (1 mark) (1 mark) - To about 2 cm3 of liquid Q in a test tube, add 2 or 3 drops of acidified potassium dichomate(VI) and warm the mixture.
Observations Inferences Orange colour persists
OR
No green colour formedAlcohol absent
OR
R-OH absent(1 mark) (1 mark)
- Place 2 drops of liquid Q on a watch glass. Ignite the liquid with a Bunsen bumer flame.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download KCSE 2019 Chemistry Paper 3 Questions and Marking Schemes.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students