SECTION A: BIOLOGY (34 marks)
Answer all the questions in this section in the spaces provided.
-
- Name two types of respiration that take place in an animal tissue. (2 marks)
- State two characteristics of living organisms that are specific to plants only. (2 marks)
- Explain three features of respiratory surfaces that adapts them to their function. (3 marks)
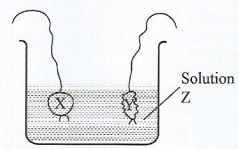
- The diagram below illustrates an experiment used to demonstrate water relationship in plant cells. X and Y are solutions in bags whose walls are semi-permeable.
- Identify the solution which is hypotonic to the liquid in the beaker. (1 mark)
- Account for the observation made in the bag containing solution X. (2 marks)
-
- Explain the presence of non-return valves in the veins of a mammalian circulatory system. (2 marks)
- Name two proteins in the blood which are responsible for determining the blood group of a person. (2 marks)
- Explain why glucose and protein are absent in urine of a healthy person. (2 marks)
- The diagram below represents a cell organelle.
- Identify the organelle. (1 mark)
- How is the part labelled K adapted to its function? (2 marks)
- State three roles of saliva in the human digestive system. (3 marks)
- Study the equation below and answer the questions that follow.
- Identify processes A and B. (2 marks)
- In which cell organelle does process B take place? (1 mark)
-
- Name the condition associated with the presence of sugar in urine. (1 mark)
- Name the two hormones involved in regulation of blood sugar in humans. (2 marks)
- State two functions of the liver in humans. (2 marks)
-
- Explain the following terms as used in blood transfusion:
- Universal donor (1 mark)
- Universal recipient (1 mark)
- State the branch of biology that deals with plants. (1 mark)
- State the role of ribosomes in the cell. (1 mark)
- Explain the following terms as used in blood transfusion:
SECTION B:CHEMISTRY (33 marks)
Answer all the questions in this section in the spaces provided.
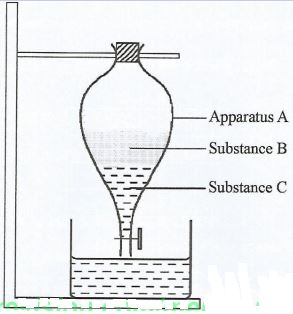
- Figure 1 is a setup to separate a mixture of substances B and C. Study it and answer the questions that follow.
- Identify apparatus A. (1 mark)
- State two properties that make substances B and C to separate. (2 marks)
- Fill in the spaces provided in Table 1. (2 marks)
Table 1
Name of the element Chemical symbol Sodium Hg -
- Element X and Y are alkali metals. X is above Y in the periodic table. Explain the differences in their ionisation energies. (X and Y are not the actual symbols of the elements). (2 marks)
- The atomic number of element W is 13. Give the period and group to which the element belongs.
- Period (1 mark)
- Group (1 mark)
-
- Define the term mass number. (1 mark)
- Use Table 2 to answer the question that follow.
Table 2
Identify the substance which is an electrolyte. (1 mark)Substances Urea Sugar Lemon
- Burning sodium metal was lowered into a gas jar containing a green gas E. A white solid F was formed.
- Identify:
- Gas E (1 mark)
- Solid F (1 mark)
- Name the product formed if sodium metal was replaced with zinc metal. (1 mark)
- Write a chemical equation for the reaction taking place between zinc and gas E. (1 mark)
- Identify:
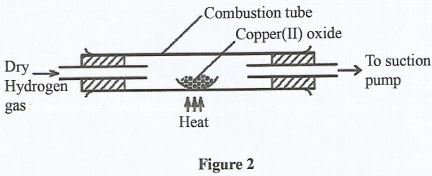
- Study the setup in Figure 2 and answer the questions that follow.
- State two observations made in the combustion tube (2 marks)
- Give the chemical property of hydrogen gas in the setup (1 mark)
-
- What is an acid-base indicator? (1 mark)
- Complete the following general equation. (1 mark)
Metal + Dilute Acid →
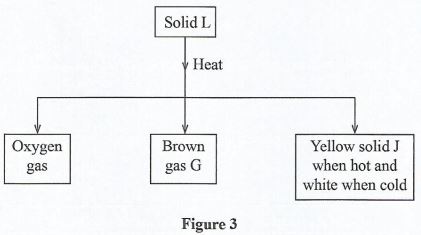
- Study the flow chart in Figure 3 and answer the questions that follow.
- Identify
- Solid L (1 mark)
- Solid J. (1 mark)
- Write a balanced chemical equation for the decomposition of solid L. (1 mark)
- Identify
-
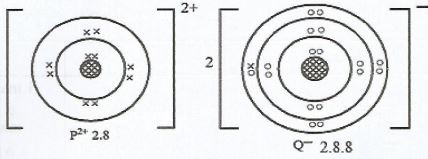
- Using dot (•) and cross (x) diagram, illustrate the bonding formed when element P reacts with element Q. (Atomic number of P is 12 and that of Q is 17). (2 marks)
- Name the type of bond in (a) (1 mark)
-
- The following equation shows the oxidation and reduction processes of lead(II) oxide and zinc.
PbO(s) + Zn(s) → ZnO(s) + Pb (s)
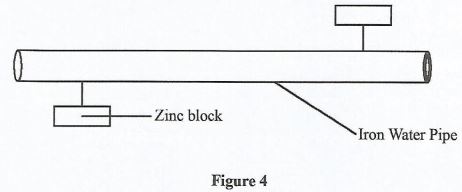
When the same reaction was repeated using magnesium oxide instead of lead(II) oxide, no product was formed. Explain. (2 marks) - The following diagram in Figure 4 shows an iron water pipe fitted with blocks of zinc.
- Name the method used to prevent rusting of the iron water pipe. (1 mark)
- Give one other method used to prevent rusting. (1 mark)
- The following equation shows the oxidation and reduction processes of lead(II) oxide and zinc.
- Table 3 shows the volume of soap used with equal volumes of water from different sources to form lather.
Table 3
Water source Volume of soap used H 12 cm3 Z 2 cm3 - Identify which water source is hard. (1 mark)
- Describe how sodium carbonate can be used to soften hard water. (2 marks)
SECTION C: PHYSICS (33 marks)
Answer all the questions in this section in the spaces provided.
- State one laboratory safety rule that must be observed by a student when operating an electrical equipment. (1 mark)
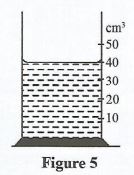
- Figure 5 shows a measuring cylinder containing a liquid of mass 40 g.
Determine:- The volume of the liquid. (1 mark)
- The density of the liquid (3 marks)
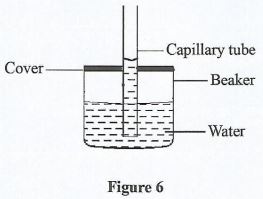
- Figure 6 shows a capillary tube dipped in water in a covered beaker.
- State the force that causes the rise of water in the tube. (1 mark)
- State what would happen to the level of water in the tube when the water in the beaker is heated for some time. (1 mark)
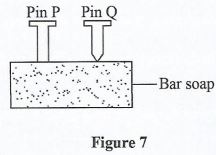
- Figure 7 shows two pins P and Q that are to be driven into a bar soap.
- Equal forces are applied on pin P and pin Q. State which pin would be more easily driven into the bar soap. (1 mark)
- Explain the reason for your answer in 25(a) (2 marks)
- Smoke particles enclosed in a transparent glass bottle are seen to be moving randomly when viewed through a microscope. Explain this observation. (2 marks)
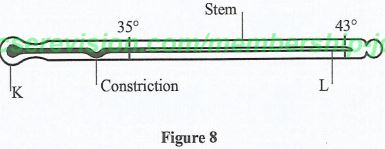
- Figure 8 shows a clinical thermometer.
- Identify the parts labelled K and L. (2 marks)
- State the reason why the scale does not go beyond 43°. (1 mark)
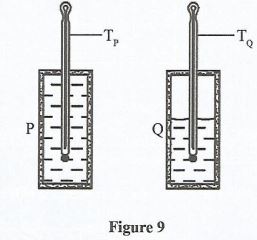
- Figure 9 shows two insulated containers fitted with a thermometer. The two containers are filled with water at 100°. Container P is filled with water to the brim while container Q is partially full.
- State the thermometer which will have the lowest reading after some time. (1 mark)
- State the reason for the answer in 28(a). (1 mark)
- A uniform metre rule weighing 6.4 N is pivoted at the 60 cm mark. Determine the point on should be suspended for the rule to be at equilibrium. thepere rule where a weight of 3.2 N should be for the rule to at be at equilibirum (4 marks)
-
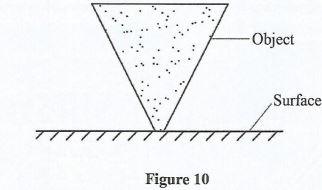
- Figure 10 shows an object resting on a flat surface.
Identify its state of equilibrium. (1 mark) - State two way Page 9 male silbility or a boy can be increased. (2 marks)
- Figure 10 shows an object resting on a flat surface.
- A student intends to make a steel spring that can support a large load without getting damaged. State three factors that the student should consider when making the spring. (3 marks)
- State the meaning of the term displacement. (1 mark)
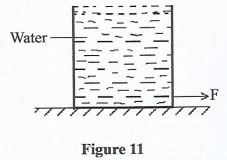
- Figure 11 shows a bucket filled with water to the brim resting on a horizontal surface.
It is observed that, when the bucket is suddenly moved forward by a force F, some water spills out in the backwards. State the reason why water spills. (1 mark) - Figure 12 shows an inclined plane being used to move a load onto a platform of height h.
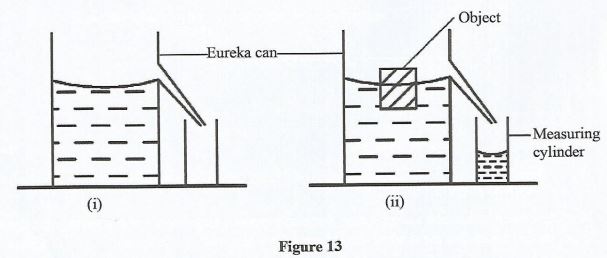
State two adjustments that can be done to ensure that a lesser force is used in moving the load onto the platform. (2 marks) - Figure 13 (i) shows a Eureka can full of water and a measuring cylinder put below the spout. When an object of weight 0.5 N is placed in water, it floats and displaces some water into the measuring cylinder as shown in figure 9(ii).
- State the weight of water in the measuring cylinders.
- State the reason for the answer in 35(a).
MARKING SCHEME
| BIOLOGY (SECTION A) | ||
| 1 |
|
(2 marks)
(2 marks) |
| 2 |
|
(3 marks) |
| 3 |
|
(1 mark)
(2marks) |
| 4 |
|
(2 marks)
(2 marks) |
| 5 |
|
(2marks) |
| 6 |
|
(1 mark) (2 marks) |
| 7 |
|
(3 marks) |
| 8 |
|
(2 marks) (1 mark) |
| 9 |
|
(1 mark) (2 marks)
(2 marks) |
| 10 |
|
(1 mark) (1 mark) |
| SECTION B: CHEMISTRY | ||
| 11 |
|
(1 mark) (1 mark) (1 mark) |
| 12 |
|
(1 mark) (1 mark) |
| 13 |
|
(2 marks)
|
| 14 |
|
(1 mark) |
| 15 |
|
(1 mark) (1 mark) |
| 16 |
|
(1 mark) |
| 17 |
|
(1 mark)
|
| 18 |
|
(1 mark) (1 mark) (1 mark) |
| 19 |
|
(1 mark)
(1 mark) |
| 20 |
|
(2 marks)
(1 mark) |
| 21 |
|
(1 mark) (2 marks) |
| 22 |
|
(1 mark) |
| 23 |
|
(1 mark)
|
| 24 |
|
(1 mark) |
| 25 |
|
(2 marks) |
| 26 |
They are being knocked by invisible air particles in constant random motion hence appears to be in constant random motion |
(1 mark) (1 mark) |
| 27 |
|
(2 mark)
|
| 28 |
|
(1 mark) (1 mark) |
| 29 | 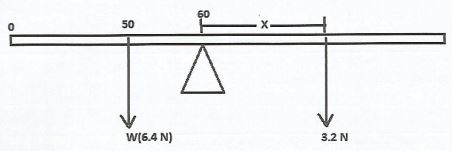 Sum of clockwise moment = Sum of anticlockwise moment 6.4 x 10 3.2 x X X = 20 cm 3.2 N should be at (100 − 20) = 80 cm mark. |
(1 mark)
(1 mark) |
| 30 |
|
(1 mark) (1 mark) |
| 31 |
|
(1 mark) |
| 32 | Distance between any two points in a speciffied direction. | (1 mark) |
| 33 | Because of inertia hence some water tends to remain behind as the bucket is pulled forward. | (1 mark) |
| 34 |
|
(1 mark) (1 mark) |
| 35 |
|
(1 mark) (1 mark) |
Download General Science Paper 1 Questions and Answers - KCSE 2021 Past Papers.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students