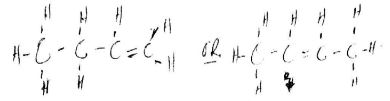QUESTIONS
-
- State one property that can be used to distinguish between a proton and a neutron.(1 mark)
- An ion of element Y has the formula:
- Write the electron arrangement of the ion. (I mark)
- Identify the group and period in the Periodic Table to which the element belongs.
Group .....................(1/2 mark)
Period. .....................(/2 mark)
-
- Complete Table 1 by writing the formula and naming the structure of the chlorides of the elements (2 marks)
Element Sodium Magnesium Silicon Phosphorus Formula of chloride Name of the Structure of chloride - Select from Table 1 an acidic chloride and write the equation for its reaction with water.(1 mark)
- Complete Table 1 by writing the formula and naming the structure of the chlorides of the elements (2 marks)
-
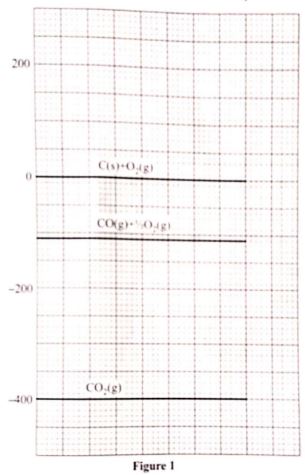
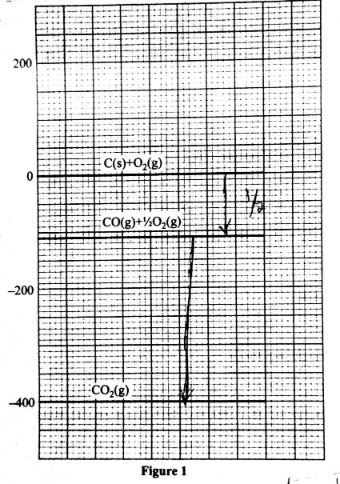
- Write a thermochemical equation for the formation of carbon(II) oxide. (1 mark)
- Use the energy level diagram in Figure 1 to answer the questions that follow.
Determine the enthalpy change of- formation of carbon(II) oxide (1 mark)
- combustion of carbon(II) oxide (1 mark)
-
- Give a reason why painting or galvanising iron sheets protects them from rusting (1 mark)
- Explain the advantage of galvanising over painting of iron sheets (2 marks)
-
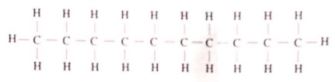
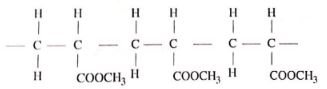
- The structure of compound A is
Give its:- name (1 mark)
- empirical formula (1 mark)
- Draw the structure of an alkanoic acid whose molecular formula is C,HO (1 mark)
- The structure of compound A is
- The following equilibrium exists in a closed system.
State and explain two conditions under which the intensity of the brown colour of the equilibrium mixture can be increased.
Condition I (11/2 marks)
Condition II (11/2marks) -
- Determine the oxidation numbers of
- hydrogen in CaH2 (1 mark)
- oxygen in OF2 (1 mark)
- Write an ionic equation for the reaction between aqueous sodium hydrogen carbonate and ethanoic acid. (1 mark)
- Determine the oxidation numbers of
- The mass of one molecule of a hydrocarbon is 9.33 x 10-23g. (Avogadro's number-6.0 x 10 mol, C-12.0, H-1.0)
- Determine its:
- molecular mass (1mark)
- molecular formula (1 mark)
- Draw a structure of the hydrocarbon in 8(a). (1 mark)
- Determine its:
-
- Water reacts with hydrogen ions:
- write the formula of the product formed (1/2mark)
- Name the type of bond formed (1/2mark)
- The melting point of iodine is higher than that of chlorine. Explain. (2 marks)
- Water reacts with hydrogen ions:
- A sample of ammonia gas can be prepared by heating a mixture of ammonium bromide and barium hydroxide.
- Write an equation for the reaction. (1 mark)
- State why the gas cannot be dried using anhydrous calcium chloride (1 mark)
- Name a suitable drying agent. (1 mark)
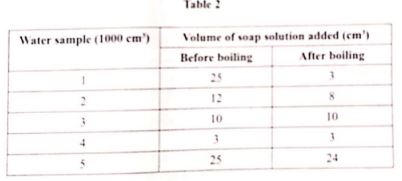
- In an experiment to test for hardness of water from different boreholes, soap solution was added to 1000 cm of water and the volume of soap solution required for lather to start forming recorded. The results are given in Table 2
- Select water samples that show:
- temporary hardness (1/2 mark)
- no hardness(1/2 mark)
- both temporary and permanent hardness (1/2 mark)
- Describe how water hardness can be removed using an ion exchange resin. (11/2 marks)
- Select water samples that show:
- Products of electrolysis at the electrodes for aqueous solutions depend on three factors. Two of these factors are concentration of electrolyte and nature of electrode
- State another factor that affects the products of electrolysis (1 mark)
- Complete Table 3 to show products of electrolysis for dilute calcium chloride and concentrated calcium chloride at the anode and cathode
(2 marks)Electrolyte Anode Cathode Dilute calcium chloride Concentrated calcium chloride
-
- Carbon exhibits different boiling points. Explain. (1 mark)
- It takes 4-4 seconds for nitrogen(IV) oxide gas to effuse through an opening. Calculate how long it will take for an equal volume of chlorine gas to effuse through the same opening (N-14.0: 0-16.0: C1-35.5). (2 marks)
-
- Give an example of a natural polymer made of
- cellulose material (1⁄2 mark)
- a hydrocarbon (1/2marks)
- Part of the structure of perspex is:
- Draw the structure of the monomer of perspex. (1 mark)
- Give two properties of perspex that make it suitable for use in making lenses. (1marks)
- Give an example of a natural polymer made of
- Two allotropes of carbon are graphite and diamond
- Explain why the density of diamond is higher than that of graphite. (1mark)
- Give one use of each of the allotropes and relate the use to properties of the allotrope
- Graphite
use (1/2mark)
property (1/2mark) - Diamond
use (1/2mark)
property (1/2 mark)
- Graphite
- The graph in Figure 2 shows radioactive decay curve of a radioactive isotope.
Use the graph to determine the:- half life of the radioactive isotope (1 mark)
- rate of decay at time 150 hours (1 mark)
- The half life of two radioactive isotopes A and B are 8 days and 5.2 years respectively Given that both of them emit beta radiation, explain why A would be more suitable in the treatment of a disease (1 mark)
- The formula of a hydrated salt of manganese is MnSO, XHO Given that the salt contains 24,7% manganese, determine the value of X (Mn-550, S-120.0-160, H1.0) (3 marks)
- Describe the correct procedure of heating a liquid in a test tube using a Bunsen burner. (3 marks)
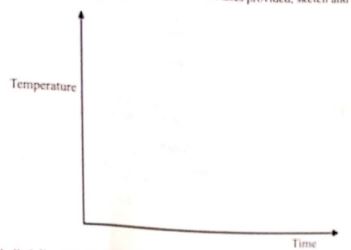
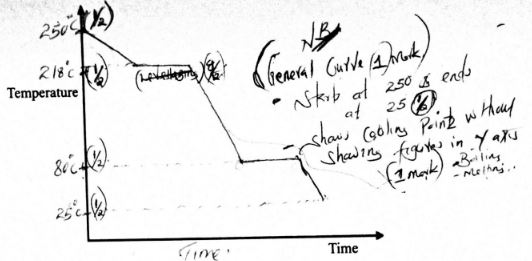
- The melting and boiling points of naphthalene are 80°C and 218°C, respectively A sample of naphthalene was cooled from 250°C to 25 °C. On the axes provided, sketch and label the cooling curve that would be obtained. (3 marks)
- Draw a labelled diagram of a setup that can be used to prepare a dry sample of chlorine gas using potassium manganate(VII) and concentrated hydrochloric acid ( marks)
- Table 4 gives the boiling points of three liquids
Describe how the following mixtures can be separatedLiquid Boiling point (C) Hexane 68.7 Butanol 99.5 Water 100.0 - hexane and butanol (11/2 marks)
- hexane and water (11/2marks)
- Complete Table 5 by writing the observations made when aqueous ammonia and aqueous sodium sulphate are added to solutions containing calcium, aluminium and iron(II) ions.(3 marks)
lons present Aqueous ammonia Aqueous sodium sulphate Ca2+ Al3+ Fe2+ -
- Iron is extracted from haematite ore. If the ore contains oxides of silicon and aluminium, explain how these impurities are removed (2 marks)
- The extraction process of iron produces waste gases. State how these waste gases can be used to lower the operational cost of the extraction process (1 mark)
- When chlorine is bubbled into a sample of water, the solution smells strongly of chlorine. If aqueous sodium hydroxide is added to the solution, the smell of chlorine disappears
The following equation shows the reaction that occurs.
With reference to the equation for the reaction, explain why the- solution smells strongly of chlorine (1 mark)
- addition of sodium hydroxide removes the smell (2 marks)
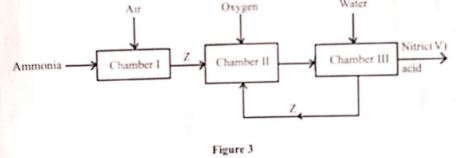
- Figure 3 shows how nitrict V) acid can be obtained
- Identify the chamber in which a catalyst is used. (1 mark)
- Name substance Z. (1 mark)
- Write an equation for the reaction that takes place in Chamber III.(1 mark)
- The fomula of complex ion formed when aqueous zinc sulphate reacts with aqueous sodium hydroxide is given as ;(Zn(OH)4)
Explain how the value of x is determined; (2marks) - Copper can be obtained from copper(ii)oxide using carbon (ii)oxide or coke .
- Name another reagent that can be used to obtain copper from copper(ii)oxide (1marks)
- The equation from the reaction with carbon(ii)oxide is ;
CuO(S) + CO(g) → Cu(s) +CO(g)
Calculate the maximum mass of copper that would be obtained using 200dm3 of carbon(ii)oxide (Cu =63.5; Molar of gas =24.0dm3) (2marks)
MARKING SCHEME .
-
- State the property that can be used to distinguish between a proton and aneutron . (1mark)
- mass
- charge
- An ion element Y has the fomula
- write the electron arrangement of the ion .
- 2. 8.8 or 2.8 .8
- Identify the group and peariod in the periodic table to which the element belongs
Group ii
period 4
- write the electron arrangement of the ion .
- State the property that can be used to distinguish between a proton and aneutron . (1mark)
-
- Complete the table by writting the fomula and naming the structure of the chloride of the elements
Element Sodium Magnesium silicon Phosphorus Formula of chloride NaCL MgCl2 SiCl4 PCl3 /PCl5
Name of the structure of chloride grant ionic Grant ionics Simple Molecular Simple molecular - Select from the table 1 an acidic chloride and write the equation for its reaction with water . (1mark)
- PCl3(l) + 3H2O(l) → H3PO3 (aq) +3HCl(aq)
PCl5(L) +4H2O(L) → H3PO4(aq) + 5HCl(aq)
- PCl3(l) + 3H2O(l) → H3PO3 (aq) +3HCl(aq)
- Complete the table by writting the fomula and naming the structure of the chloride of the elements
-
- Write a thermochemical equeation for the formation of carborn(ii)oxide (1mark)
C(s) + 1/2O2 → CO(3) ;(ΔH°f) - Use the energy level in figure 1 to answer the equation that follow.
Determine the enthalpy change of ;- formation of carbon|(ii0oxide
ΔH°f = -110ki/mol - combination of carbon(ii)oxide
ΔH -400 -(-110) =-290kj
- formation of carbon|(ii0oxide
- Write a thermochemical equeation for the formation of carborn(ii)oxide (1mark)
-
- Give a reason why painting ir galvanising iron sheets protects them from rusting .
- both methods provides coating /that keeps iron from oxygen and water
- Explain the advantage of galvanising over painting if iron sheets (2marks)
- In galvanising , zinc acts as sacrificial metal source
- it is more reactive than iron thus prevent rusting ,
- Give a reason why painting ir galvanising iron sheets protects them from rusting .
-
- The structure of compound A is ;
Give its ;- name
Decane (1mark) - emperical fomula
C5H4 (1mark)
- name
- Draw the structure of an alkanoic acid whose moleccular formula is C4H10O2 (1mark)
- The structure of compound A is ;
- The following equilibrium exists in a closed system
state and explain two conditions under which the intensity of the brown colour of the equilibrium mixture can be increased .
conditon 1
increase temperature ,1/2 the forward reaction is endothermic / form of NO2(g) is formed by increase in temperature
conditon II
Deduction in pressure ,1/2 forward reaction proceeds with increase in number of pressure .production od NO2 is there for fevoured by low pressure -
- Determine the oxidation number of ;
- hydrogen in CaH2 (1mark)
O.N of CaH2 = +2+2H =0(1/2)
2H = -2
O°NO of H =-1 (1/2) - oxygen in Of2
O'No of OF2 = O +2(-1)
O= +2
- hydrogen in CaH2 (1mark)
- Write an ionic equation for the reation between aqueous sodium hydrogen carbonate and ethanoic acid . (1mark)
- CH3COOH(aq) = HCO3‾(aq) → CH3COO‾(aq) =CO2 +HO2
- Determine the oxidation number of ;
- The mass of one molecule of a hydrocarbon is 9.33 ×10-23g. (Avogadro's number = 6.0×1023 mol-1 , C = 12.0;H =1.0)
- Determine its:
- molecular
9.33 × 10-23× 6.0 × 10 - molecular formula (1mark)
C4 H8
- molecular
- Draw a structure of the hydrocarbon in 8 (a) (1mark)
- Determine its:
-
- Water reacts with hydrogen ions :
- Write the chemical fomular of the product formed (1/2 mark)
H3O+ - Name the type of bond formed (1/2marks)
Co-ordinate
- Write the chemical fomular of the product formed (1/2 mark)
- The melting point of iodine is higher than that if chlorine . Explain (2marks)
- Iodine has larger molecular mass the stronger vender walls forces of attractions then chlorine which has smaller mass .
- Water reacts with hydrogen ions :
- A sample of ammonia gass can be prepared by heating a mixture of ammonia bromide and barium hydroxide .
- Write an equation for the reaction
2NH4Br(s) + Br(OH)2(s) → BaBr2(s) + 2NH3(g) +2H2O4(g) - State why the gas cannot be dried using anhydrous calcium chloride (1mark)
- Ammonia reacts with calcium chloride to form CaCl2•2NH3 which is a complex salt .
- Name a suitable drying agent .
- CaO(s)/Calcium oxide
- Write an equation for the reaction
- In an experiment to test for hardness of water from different boreholes , soap solution was added to 1000cm³ of water and the volume od soap solution required for lather to start forming recorded. The results are given in table 2
- Select water sample that shows :
- temporary hardness (1/2marks)
- Sample 1
- no hardness (1/2marks)
- Sample 4
- both temporary and parmanent hardness (1/2marks)
- Sample 2
- temporary hardness (1/2marks)
- Describe how water hardness can be removed usingind an ion exchange resin (11/2marks)
- Hard water is run into a column containing the ion exchange resin
- Ca2+ and Mg2+ ion arte exchanged for Na+ ion
- therefore water coming out is soft .
- Select water sample that shows :
- Product of electrolysis at the electrode for aqueous solution depend on the factors . Two of these factors are concentration of electrolyte and of electrode.
- State another factor that affects the product of electrolysis (1mark)
- Position of the ion/element in the ractevity searies
- Complete the table 3 to show products of electrolysis for dilute calcium chloride and concentrated calcium chloride at the anode and cathode. (2marks)
Electrolyte anode Cathode Dilute calcium chloride Oxygen Hydrogen
Concentrated calcium chloride Chloride Hydrogen
- State another factor that affects the product of electrolysis (1mark)
-
- Carbon exhibits different boiling points . Explain. (1mark)
- Carbon exist in defferent crystoline forms same physical state hence different boiling point because of their different structure.
- It takes 4-4 seconds for nitrogen(IV) oxide gas to effuse through an opening. Calculate how long it will take for an equal volume of chlorine gas to effuse through the same opening (N-14.0: 0-16.0: C1-35.5). (2 marks)
- Carbon exhibits different boiling points . Explain. (1mark)
-
- Give a example of a natural polymer made of :
- cellulose material (1/2marks)
- Cotton wool ,wood ,paper
- a hydrocarbon (1/2marks)
- Rubber
- cellulose material (1/2marks)
- Part of the structure of perspex is
- Draw the structure of the monomer of perspex. (1 mark)
- Give two properties of perspex that make it suitable for use in making lenses . (1mark)
- Transparent - free from change of refraction index
- strong -consistant in refraction index
- Draw the structure of the monomer of perspex. (1 mark)
- Give a example of a natural polymer made of :
- Two allotropes of carbon are graphites and diamond .
- Explain why the desity of diamond is higher than that of graphite . (1mark)
Diamon has tetrahedral structure with all atoms forming four cobalent bond while in the graphite each atom form three covalent bonds in a layer structure which are far from each other . - Give one use of each of the alltropes and relate the use to propertiesof the allotrope.
- Graphite
use (1/2mark)
- Lubricant / Electrode
property (1/2mrks) - conduct of electricity
- Lubricant / Electrode
- Diamond
use (1/2mark)- tips od driling tips
property (1/2marks) - Hardand abrasive
- cutting instrument
- tips od driling tips
- Graphite
- Explain why the desity of diamond is higher than that of graphite . (1mark)
-
- The graph in fugure 2 shows a radioactive decay curve of a radioactive isotope
.
Use the graph to determine the ;- half life of the radioactive isotope (1mark)
- Half ; life = 85 hours
- rate of decay at time 150hours (1mark)
3.0 - 0.5/80 -240
-0.016g/hrs
- half life of the radioactive isotope (1mark)
- The half life of two radioactive isotopes A and B are 8 days and 5.2 years respectively Given that both of them emit beta radiation, explain why A would be more suitable in the treatment of a disease (1 mark)
- A has a shoter half-life the B will clear from the body faster thus not expose the patient to radiation for a long time
- The graph in fugure 2 shows a radioactive decay curve of a radioactive isotope
- The formula of a hydrated salt of manganese is MnSO, XHO Given that the salt contains 24,7% manganese, determine the value of X (Mn-550, S-120.0-160, H1.0) (3 marks)
- Describe the correct procedure of heating a liquid in a test tube using a Bunsen burner. (3 marks)
- Hold the test tube with a test tube holder , keep it slanting with the open end facing away ,heat from top downwards and not from bottom to the top while rotating
- The melting and boiling points of naphthalene are 80°C and 218°C, respectively A sample of naphthalene was cooled from 250°C to 25 °C. On the axes provided, sketch and label the cooling curve that would be obtained. (3 marks)
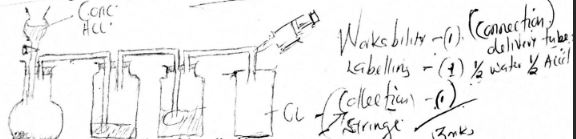
- Draw a labelled diagram of a setup that can be used to prepare a dry sample of chlorine gas using potassium manganate(VII) and concentrated hydrochloric acid ( marks)
- Table 4 gives the boiling points of three liquids
Describe how the following mixtures can be separatedLiquid Boiling point (C) Hexane 68.7 Butanol 99.5 Water 100.0 - hexane and butanol (11/2 marks)
- Fraction distilation
put the two liquidin fractionating column ;heat the mixture gently ;hexone will distill ;
- Fraction distilation
- hexane and water (11/2marks)
- separating funnel ;burette
this are immiscible liquids ;hexane will float on water from the bottom of flask , hexane remove in the funnel
- separating funnel ;burette
- hexane and butanol (11/2 marks)
- Complete Table 5 by writing the observations made when aqueous ammonia and aqueous sodium sulphate are added to solutions containing calcium, aluminium and iron(II) ions.(3 marks)
lons present Aqueous ammonia Aqueous sodium sulphate Ca2+ white precipitate white precipitate Al3+ white precipitate No white precipitate Fe2+ Green precipitate No Green precipitate -
- Iron is extracted from haematite ore. If the ore contains oxides of silicon and aluminium, explain how these impurities are removed (2 marks)
- they react with calcium oxide ;to form CaSiO3 whic are removed as slag
- The extraction process of iron produces waste gases. State how these waste gases can be used to lower the operational cost of the extraction process (1 mark)
- The waste gase are at high temp , the head can be recycle to pre-heat incoming air
- Iron is extracted from haematite ore. If the ore contains oxides of silicon and aluminium, explain how these impurities are removed (2 marks)
- When chlorine is bubbled into a sample of water, the solution smells strongly of chlorine. If aqueous sodium hydroxide is added to the solution, the smell of chlorine disappears
The following equation shows the reaction that occurs.
With reference to the equation for the reaction, explain why the- solution smells strongly of chlorine (1 mark)
- chlorine reacts partialy with water , there is strong smell due to presence of chlorine molecules .
- addition of sodium hydroxide remove the smell (2marks)
- Addition of NaOH neutralize HCl (aq) and HOCl(aq) ,equilibrium shifts to the right chlorine molecule are consum hence the smell disappears.
- solution smells strongly of chlorine (1 mark)
- Figure 3 shows how nitrict V) acid can be obtained
- Identify the chamber in which a catalyst is used. (1 mark)
- chamber 1
- Name substance Z (1mark)
- Nitrogen (ii)oxide (No)
- Write an equation for the reaction that takes place in chamber III (1mark)
- 3NO2(g) + H2O(l) → 2HNO3(aq)
- Identify the chamber in which a catalyst is used. (1 mark)
- The fomula of complex ion formed when aqueous zinc sulphate reacts with aqueous sodium hydroxide is given as ;(Zn(OH)4)
Explain how the value of x is determined; (2marks)- Oxidation state of zinc is =2
- OH‾ has a charge of -1
- +2 + (-1× 4) = X
- x=-2
- Copper can be obtained from copper(ii)oxide using carbon (ii)oxide or coke .
- Name another reagent that can be used to obtain copper from copper(ii)oxide (1marks)
- hydrogen gas
- The equation from reaction with carbon(ii)oxide is
- CuO(s) + CO(g) → Cu(s) + CO2(g)
Calculate the maximum mass of copper that would be obtained using 200dm3 of carbon(ii)oxide (Cu =63.5; Molar of gas =24.0dm3) (2marks)
CuO(s) + CO(g) → Cu2(g) ,
Mole CO= mole cu =200dm3/24dm3
mass =200/24 × 63.5 =529.2
- CuO(s) + CO(g) → Cu(s) + CO2(g)
- Name another reagent that can be used to obtain copper from copper(ii)oxide (1marks)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download CHEMISTRY Paper 1 Questions and Answers - KCSE 2022 Past Papers.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students