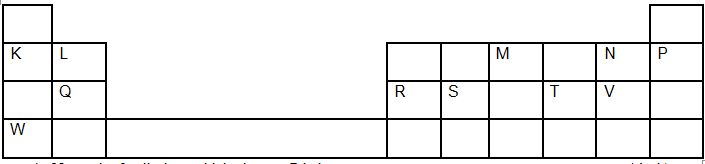
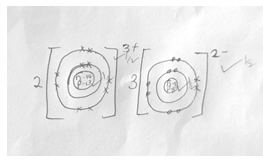
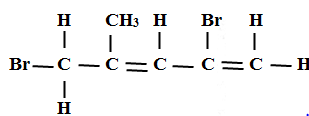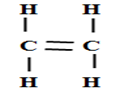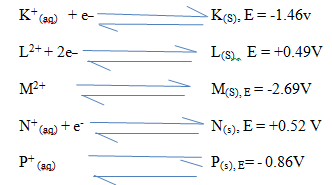- The grid below shows a section of the periodic table, the letters are not the actual chemical symbol.
- Name the family into which element P belongs to ( 1mk)
- Which two elements forms the most soluble carbonates (2mks )
- With a reason, identify elements in period 3 with the largest atomic radius (2mks )
- Write the formula of the compound formed between Q and M (1mk )
- State two uses of element R and for each use , state property of element R that makes lts possible for the use
- Use ( 1mk)
Property (1mk) - Use (1mk)
Property (1mk)
- Use ( 1mk)
- Using dots and cross ,show bonding in the compound formed between R and oxygen (2mks )
- In terms of structure and bonding explain why the oxides of element Thas relatively low boiling points (2mks)
-
- name the following compounds (3mks)
- CH3CH2CH2COOH
-
- CH3CH2OOCCH2CH3
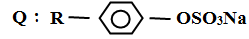
- Two types of detergents P and Q can be represented as
P: R - COONa- Identify each type of the detergent (2mks)
- Which of the two detergents is the best to use with hard water? Give a reason(2mks)
- State one advantage of detergent P (1mk)
- State one disadvantage of detergent Q (1mk)
- An hydrocarbon can be represented as follows
- Identify the hydrocarbon (1mk)
- Name two reagents that can reacted together to generate the hydrocarbon (2mks)
- name the following compounds (3mks)
-
- Name two apparatuses that can be used for determining mass in a laboratory (2mks)
- One of the flames produced by Bunsen burner is the luminous flame
- Explain why this flame is very bright (1mk )
- State two disadvantages of the luminous flame (2mks)
- Air is usually one of the substances that is considered as a mixture
- Identify the two most abundant component of air (2mks )
- Give two reasons why the air is considered as a mixture (2mks)
- One of the components of air is carbon (iv) oxide. Describe an experiment that can be used to prove the presence of carbon (iv) oxide in the air (2mks)
-
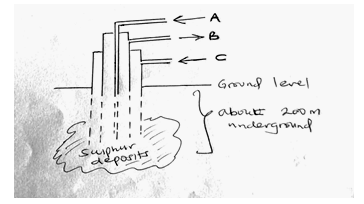
- The diagram below shows the process used to obtain Sulphur from underground deposits
- Name the above process used to obtain sulphur from the underground deposits (1mk)
- Name the substance passed through pipe
A (1mk)
B (1mk) - State two properties of Sulphur that makes it possible to extract using the above process (2mks)
- The diagram below shows the contact process used in the manufacture of concentrated sulphuric(vi) acid Identify the following:
- Substance Q formed in the burner (1mk)
- Chamber T (1mk)
- Substance R (1mk)
- Substance S (1mk)
- Write the chemical equation occurring in the dilution chamber (1mk)
- Why is it necessary to pass substance Q though a purifier (1mk)
- State one use of sulphuric (VI) acid (1mk)
- The diagram below shows the process used to obtain Sulphur from underground deposits
-
- Calamine is one of the ores from which zinc can be extracted from
- Name any other ore from which zinc can be extracted from (1mk)
- The calamine is usually decomposed by heating to obtain substance M as shown below
ZnCO3 →M + CO2
Identify substance M (1mk) - Identify two methods that can be used to obtain zinc from substance M (2mks)
- During the extraction of zinc, name two gases likely to emitted into the air and that are likely to cause pollution (2mk)
- State one likely pollution effects of each of the gases you have mentioned in (a) above (2mks)
- State one possible use of zinc metal (1mk)
- Calamine is one of the ores from which zinc can be extracted from
-
- define the term electrolysis (1mk)
- State two functions of a salt bridge during electrolysis (2mks)
- The reduction potential of elements K, L, M, and P are as given below.
- Which letter represents the, strongest reducing agent? give a reason (2mks)
- Which two letters represent elements whose half cells would form an electrochemical cell with the largest e.m.f? (1mk)
- Calculate the e.m.f of the cell formed in (ii) above (2mks)
- During the electrolysis of a molten chloride of metal Q, a current of 0.25A was passed though the molten chloride for 2 hours and 10minutes. Given that 0.9grams of metal Q were deposited at the cathode.
- Calculate the quantity of electricity passed (1mk)
- Charge carried by the ions of metal Q given that R.A.M of metal Q is 84 (3mks)
-
- starting with magnesium oxide, describe how you can obtain a dry sample of magnesium Carbonate (3mks)
-
- Give one example of an acid salt ( 1mk)
- When sodium nitrate was heated a solid A and gas B were produced identify solid A and gas B (2mks)
- State two uses of gas B produced in (ii) above (2mks)
- State two factors that should be considered when choosing a fuel (2mks)

MARKING SCHEME
-
- Noble gases
- K and W
- Q, it has lowest nuclear charge hence electrons in the energy level are least pulled towards the nucleus
- Q3M2 or Mg3N2
- Used for making sufuria /cooking pan
Property – good conductor of heat
Used for making overhead cables
Property – not easily corroded / good conductor of electricity (penalize electrical cables) -
- It has molecular structure with weak van der waals forces of attraction between the molecules which require little energy to break.
-
-
- Butanoic acid
- 2,5 _ dibromo -4-methylpent-1,3-diene
- Enthyl propanoate
-
- P-soapy detergent
Q – soaplesss detergent - Q – does not form scum with hard water or it lathers easily
- It is biodegradable
- It is non-biodegradable hence pollutes the environment
- P-soapy detergent
-
- Ethene
- Ethanol and concentrated sulphuric (VI) acid
-
-
- Top pan balance
Electronic balance
Beam balance -
- Due to incomplete combustion, it produces white hot carbon particles that emittes a lot of light
- It produces soot that makes apparatus dirty
It does not produce much heat
- Nitrogen and oxygen
It can be separated by physical means
Components of air are not chemically combined
Pass air through lime water (Ca(OH)2) the lime water forms white precipitate indicating presence of carbon(IV)oxide
- Top pan balance
-
- Frasch process
A – hot compressed air
C – super heated water
It has low boiling point
It is insoluble in water - Sulphur (IV)oxide
Catalytic chamber
Concentrated sulphuric (VI)acid
Water -
- H2S2O7(l) + H2O(l) →H2SO4(l)
- To remove impurities which may poison the catalyst
- Manufacture of fertilizer
Manufacture of detergent
Manufacture of dyes and paints
Used in lead acid accumulators
(any one correct)
- Frasch process
-
- Zinc blende (penalize zinc sulphide )
ZnO
Reduction using carbon or carbon (II) oxide
It is converted to zinc sulphate and electrolyzed - Sulphur {IV} oxides/SO2
Carbon {IV} oxide /CO2 - Sulphur {IV} oxide leads to formation of acid rain
Carbon oxide causes global warming - Zinc is used to galvanise iron to prevent it form rusting
To make brass an alloy of copper and zinc
{any one correct}
- Zinc blende (penalize zinc sulphide )
-
- Electrolysis is the chemical decomposition of an electrolyte using electrical energy
- Complete the circuit by making contact between the two solutions
Maintains balance of charges in electrolytes by providing ions to replace those that are used up or those that are formed -
- M – it has the most negative value
- M and N
- E =E R-h-s
+0.52 – [-2.69]
=+3.21v - Q = 1 t
=0.25 x 130x60
=1950C -
-
- Add magnesium oxide to HNO3/HCL/H2SO4 till in excess
-Filter to obtain the filtrate
Add Na2CO3[any soluble carbonate] solution
-Filter to obtain insoluble magnesium carbonate
-Rinse and dry between filter papers -
- NaHSO4 /KHSO4
- Solid A – NaNO2/Sodium nitrate
Gas B – O2/oxygen - Mixed with helium is used by mountain climbers and deep sea divers
Air enriched with oxygen is used in hospitals by patients with breathing difficulties
- Availability
Cost of fuel
Heating value
{any two correct}
- Add magnesium oxide to HNO3/HCL/H2SO4 till in excess
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download CHEMISTRY PAPER 2 - KCSE 2019 ALLIANCE MOCK EXAMINATION.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students