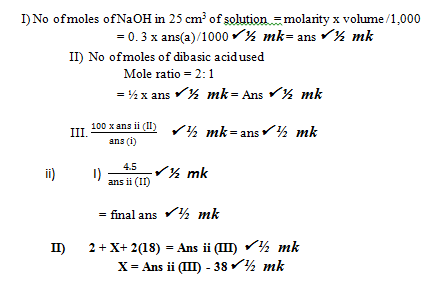INSTRUCTIONS TO CANDIDATES.
- Answer all the questions
- You are not allowed to start working with the apparatus for the first 15 minutes of the 2 ¼ hours allowed in this paper.
This is to enable you read the question paper and make sure you have all the chemicals and apparatus you may need. - Mathematical tables and Electronic calculators may be used.
- All working must be clearly shown where necessary.
- You are provided with:
- 5g of dibasic acid H2X. 2H2O, Solid A
- Solution B, 0.4M sodium hydroxide.
You are required to: - You are required to determine the solubility of Solid A in water at different temperatures.
- Determine RAM of dibasic A in H22H2O.
Procedure I:
- Place all solid A into a clean boiling tube. Using a burette, add 4cm3 of distilled water to solid A in the boiling tube. Heat the mixture while stirring with a thermometer to about 70o When all the solid has dissolved allow the solution to cool while stirring with the thermometer. Note the temperature (Ts) at which crystals of solid A first appear. Record this temperature in table I
- Using the burette, add 2cm3 of distilled water to the contents of the boiling tube, warm the mixture while stirring with the thermometer until ALL the solid dissolves. Allow the mixture to cool while stirring. Note and record the temperature at which crystals of solid A first appear.
- Repeat procedure (b) two more times and record the temperatures in table I.
Retain the contents of the boiling tube for use in procedure (II) - Complete table I by calculating the solubility of solid A at different temperatures.
NOTE:
You may hasten cooling for the first two temperatures readings by pouring cold water from the tap on the sides of the boiling tube.- TABLE I (6mks)
Volume of water added (cm3)
4
6
8
10
Crystallization temperature, Ts (°C)
Solubility of solid C in g/l00g water
- Plot a graph of solubility of solid A with crystallization temperature Ts. [3mks]
- Use your graph to determine
- the solubility of solid A in water at 55°C. [1 mk]
- determine the temperature at which 100g of solid A would dissolve in 100 cm3 of water. [1 mk]
- TABLE I (6mks)
Procedure II
Transfer the content of the boiling tube into 100ml Measuring cylinder. Rinse both the boiling tube and thermometer with distilled water and add to the measuring cylinder and shake thoroughly. Add more water carefully to make up to 100 ml mark. Label this solution A. Fill the burette with solution A (H2X. 2H2O). Pipette 25cm3 of solution B into a conical flask. Add 2-3 of Phenolphthalein indicator and titrate with solution A. Record your readings in table II below. Repeat the procedure and complete table II.
Table II (4mks)
|
I |
II |
III |
|
|
Final burette reading (cm3) |
|||
|
Initial burette reading (cm3) |
|||
|
Volume of solution A used (cm3) |
- Calculate the average volume of solution A used. (1 mk)
- Calculate the ;
- Number of moles of sodium hydroxide in 25 cm3 solution B. ( 1mk)
- number of moles dibasic acid solution A used, given the equation for the reaction as;
2NaOH +H2X →Na2X + 2H2O (1 mark) - number of moles of dibasic acid in 100cm3 of solution A 1 mark
- Determine the ;
- Relative formula mass of dibasic acid, H2X.2H2O. 1 mrk
- RAM of X in dibasic acid, H2X.2H2O. (H=1,O=16) 1 mark
- You are provided with solid E containing two cations and one anion. Carry out the tests given and record your observations and deductions in the space provided.
- Place half of solid E in a clean dry test-tube and heat gently then strongly. Test any gases produced with both blue and red litmus papers.
Observation
Deductions
(2 mks)
( 1 mk)
- Place the remaining solid E into a boiling tube. Add about10cm3 of distilled water and shake thoroughly. Divide the resultant mixture into 4 portions.
- To the first portion add a few drops of sodium hydroxide solution till in excess.
Observation
Deductions
( 1 mk)
( 1 mk)
- To the second portion, add a few drops of ammonium hydroxide solution till in excess.
Observation
Deductions
( 1 mk)
( 1 mk)
- To the third portion, add 2-3 drops of dilute hydrochloric acid.
Observation
Deductions
( 1 mk)
( 1 mk)
- To the third portion, add 2-3 drops of Lead (II) nitrate solution.
Observation
Deductions
( 1 mk)
( 1 mk)
- To the third portion, add a few drops of Barium chloride solution.
Observation
Deductions
( 1 mk)
( 1 mk)
- To the first portion add a few drops of sodium hydroxide solution till in excess.
- Place half of solid E in a clean dry test-tube and heat gently then strongly. Test any gases produced with both blue and red litmus papers.
- You are provided with solid F. Carry out the tests below. Record your observations and inferences in the spaces provided.
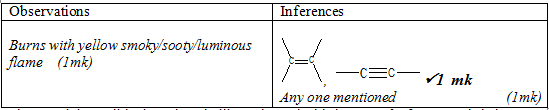
- Place about half of solid F on a metallic spatula and burnt it using a non-luminous flame
Observations
Inferences
(1mk)
(1mk)
- Place the remaining solid F in a clean boiling tube and add about 5cm3 of water and shake thoroughly.
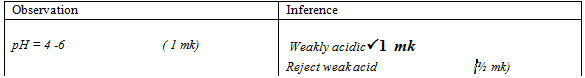
- To about 2cm3 of the solution F, put the universal indicator paper provided to determine its PH.
Observations
Inferences
(1mk)
(1mk)
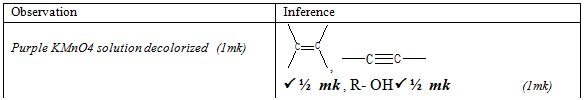
- To about 2cm3 of solution F, add three drops of acidified potassium manganate (VII) solution and warm.
Observations
Inferences
(1mk)
(1mk)
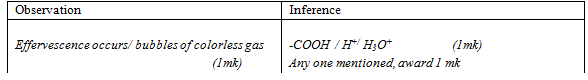
- To about 2cm3 of solution F, add solid sodium hydrogen carbonate.
Observations
Inferences
(1mk)
(1mk)
- To about 2cm3 of the solution F, put the universal indicator paper provided to determine its PH.
- Place about half of solid F on a metallic spatula and burnt it using a non-luminous flame

MARKING SCHEME
-
- TABLE I (6marks)
Volume of water in boiling tube(cm3)
Temperature at which crystals of solid A first appear (0C)
Solubility of solid A (g/100g of water)
4
66
112.5
6
56
75.0
8
49
56.0
10
44
45.0
- Complete table.........1mk
complete table 1 mark otherwise penalise fully
Penalise ½ mk if all temperature readings given in the table are constant.
For initial temp, treat temp. below 40oC and those above 80o C as unrealistic and penalise ½ mk once. - Use of decimals......... (Tied temperature readings)................. 1mk
Accept ONLY if all readings are recorded CONSISTENTLY as whole numbers or ONE decimal point of .0 or .5 other wise penalise fully. - Accuracy (AC)........ 1mk
Compare candidates first temperature reading with the SCHOOL VALUE / CENTRE VALUE. If within + 2oC of the S.V /C.V award 1 mk otherwise penalise fully.
Award 1mk for continuous decrease in the temperature. - solubility calculation............2mks
Award ½ mk for each value of solubility correctly calculated
- Complete table.........1mk
- Graph .................. 3mks
Marks distributed as follows- L.A ................................................ ½ mk
Conditions / Penalties
Penalise fully for inverted axis.
Penalise fully for wrong units: if no units are given ignore and award fully .
If only one axis is labelled / units given, condition (ii) above is applied. - Scale.......................................................½ mk
- Area occupied by the ACTUAL plots MUST be at least ¾ of the graph paper provided.
- Scale intervals MUST be constant / consistent.
- The scale chosen must be able to accommodate all the plots / points.
- Plotting ................................................. 1mk
- Award 1mk if 4 points are correctly plotted.
- Award ½ mk if only 3 points are correctly plotted: otherwise if less than 3points are plotted correctly: award 0 mk.
- If scale intervals are inconsistent then accept plots if any within the FIRST interval only.
- Accept plots even if the axis are inverted and award accordingly.
- shape/ curve...............................................................1mk
Award 1mk for descending curve/ line passing through all the plotted points.
Otherwise penalise fully.
- L.A ................................................ ½ mk
-
- Showing on the graph ½ mk
Correct answer (630C+1) ½ mk - Showing on the graph ½ mk
Correct answer (72+1g/100g H2O) ½ mk
TABLE II (5 marks )
CT lmkI
II
III
Final burette reading (cm3)
15.0
15.0
15.0
Initial burette reading (cm3)
0.0
0.0
0.0
Volume of solution A used (cm3)
15.0
15.0
15.0
DP lmk
AC 1mk
PA 1mk
FA lmk- Complete table: penalize to a maximum of ½ mk for:
- Inverted table
- Wrong arithmetic
- Burette readings beyond 50cm3 except where explained
- Unrealistic titre values (below 1cm3) and above 50cm3
- Use of decimals
- One decimal or 2 decimal places throughout otherwise penalize fully
- For use of 2 decimal places, the 2nd digit after the decimal is either ‘0’ or ‘5’ otherwise penalize fully.
- Accuracy
- Compare any of the teachers’ titre value. If any
- Within ±0.1 of T.V...lmk
- Within ±0.2 of T.V. ½ mk
- Non-within ±0.2 of T.V....Omk
- Averaging
- If 3 Averaged and within ±0.2 of each... lmk
- If 2 averaged and within ±0.2 of each....1 mk
- Otherwise penalize fully for averaged values outside ±0.2 of s.v
- Final answer
- Compare teacher’s averaged titre
- If within±0.1 of T. average titre....lmk
- If within ±0.2 of T. average titre.. ..½mk
- If outside of ±0.2 of T. averaged titre 0mk
- Complete table: penalize to a maximum of ½ mk for:
- Average volume= V1+V2+V3 (transferred to table II as shown above)
3
Calculations- No of moles of NaOH in 25 cm3 of solution = molarity x volume /1,000
= 0. 3 x ans(a) /1000 ½ mk = ans ½ mk - No of moles of dibasic acid used
Mole ratio = 2: 1
= ½ x ans P ½ mk = Ans P ½ mk -
- No of moles of NaOH in 25 cm3 of solution = molarity x volume /1,000
- Showing on the graph ½ mk
- TABLE I (6marks)
- You are provided with solid E containing two cations and one anion. Carry out the tests given and record your observations and deductions in the space provided.
- Place half of solid E in a clean dry test-tube and heat gently then strongly. Test any gases produced with both blue and red litmus papers.
Observation
Deductions
-Blue litmus remains blue
-Red litmus turns blue½ mk
-Colourless liquid forms on cooler parts½ mk
-White residue ½ mk
-NH4+ (tied to Red litmus turns blue) ½ mk
-Hydrated salt (tied to Colourless liquid forms on cooler parts) ½ mk
- Place the remaining solid E into a boiling tube. Add 10cm3 of distilled water and shake thoroughly. Divide the resultant mixture into 4 portions.
- To the first portion add a few drops of sodium hydroxide solution till in excess.
Observation
Deductions
White precipitate ½ mk dissolves in excess½ mk
Zn2+, Al3+, Pb2+
3 mentioned 1 mk
2 mentioned ½ mk
1 mentioned 0 mk
Penalize fully for any contradictory ion - To the second portion, add a few drops of ammonium hydroxide solution till in excess.
Observation
Deductions
White precipitate ½ mk insoluble in excess ½ mk
Al3+, Pb2+
2 mentioned P1 mk
1 mentioned ½ mk
Penalize fully for any contradictory ion - To the third portion, add 2-3 drops of dilute hydrochloric acid.
Observation
Deductions
No White precipitate 1 mk
No effervescencePb2+ absent
SO 3 2- , CO 3 2- absent - To the third portion, add 2-3 drops of Lead (II) nitrate solution.
Observation
Deductions
White precipitate 1 mk
SO4 2- ,Cl-
2 mentioned 1 mk
1 mentioned ½ mk
Penalize fully for any contradictory ion - To the third portion, add a few drops of Barium chloride solution.
Observation
Deductions
White precipitate ½ mk
SO4 2- ½ mk
Penalize fully for any contradictory ion
- To the first portion add a few drops of sodium hydroxide solution till in excess.
- Place half of solid E in a clean dry test-tube and heat gently then strongly. Test any gases produced with both blue and red litmus papers.
- You are provided with solid F. Carry out the tests below. Record your observations and inferences in the spaces provided.
- Place about half of solid F on a metallic spatula and burnt it using a non-luminous flame
- Place the remaining solid F in a clean boiling tube and add about 5cm3 of water and shake thoroughly.
- To about 2cm3 of the solution F, put the universal indicator paper provided to determine its PH.
- To about 2cm3 of solution F, add three drops of acidified potassium manganate (VII) solution and warm.
- To about 2cm3 of solution F, add solid sodium hydrogen carbonate.
- To about 2cm3 of the solution F, put the universal indicator paper provided to determine its PH.
- Place about half of solid F on a metallic spatula and burnt it using a non-luminous flame
Download CHEMISTRY PAPER 3 - KCSE 2019 MASENO MOCK EXAMINATION (WITH MARKING SCHEME).
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students