- The table below gives some elements of the periodic table and their atomic masses, atomic numbers and melting points. The letters are not the actual symbols of the elements.
Element
B
C
D
E
F
G
H
I
J
K
Atomic No.
7
8
19
15
2
9
6
16
12
11
Atomic mass
14
16
39
31
4
19
12
32
40
23
Melting point (0C)
-
-
63.7
44
-272
-223
Vary
113
669
98
- Select two elements with oxidation states of -3. (1mark)
- Which elements represent the most powerful reducing agent? Explain. ( 1 mark)
- Which element has the highest ionization energy? ( 1 mark)
- Select two elements which when reacted form a compound that conducts electricity both in molten and aqueous state. (1mk)
- Select any two elements which when reacted form a compound that dissolves in water to form an acidic solution. (1mk)
- Using dots () and crosses (X) to represent electrons; draw diagrams to show bonding between B and J. (2mks)
- Explain why for some elements the atomic mass is not twice the atomic number. (1mk)
- Explain why the melting point of element K is higher than that of element D. (2mks)
- Describe how a solid mixture of the Sulphate of element K and lead (II) Sulphate can be separated. (3mks)
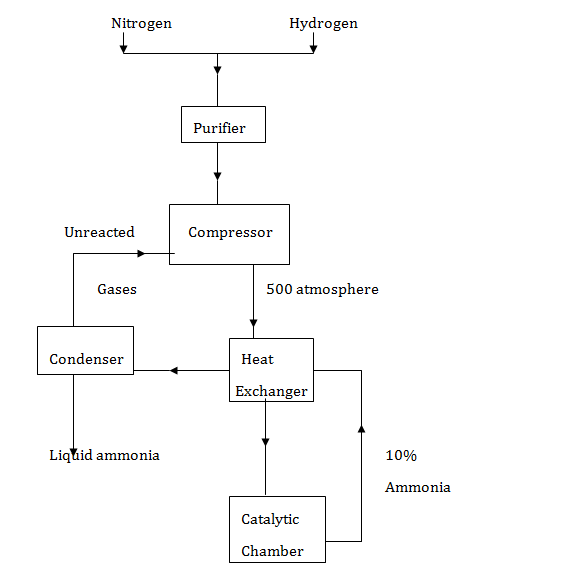
- The diagram below represents the Haber process for the manufacture of ammonia. Study it and answer the questions that follow.
- Name any two impurities removed by the purifier. (2marks)
- The catalyst used in the process is finely divided iron. Why iron is finely divided? (1mark)
- In the Haber process the conversion of nitrogen and hydrogen into ammonia is only 10%.
The remaining unreacted gases are recycled. What is the advantage of this? (1mark) - A part from iron catalyst and pressure of 500 atmospheres, name any other condition required for this process. (1mark)
- Give any two uses of ammonia (1mark)
- In the manufacture of nitric (v) acid from ammonia and air, ammonia is catalytically oxidized to nitrogen (ii) oxide
- Name the catalyst used in this reaction (1mark)
- Write a balanced chemical equation for the reaction between ammonia and air. (1mark)
- State one environmental problem likely to be faced in an area where nitric (v) acid manufacturing plant is located. (1mark)
-
- In the preparation of chlorine gas in a school laboratory, either manganese (IV) oxide or potassium manganate(VII) may be used on concentrated hydrochloric acid. State one advantage of potassium manganate (VII) over manganese (IV) oxide in this reaction. (1mark
- State and explain what would be observed when dry litmus papers are dipped in a gas jar of chlorine. (2marks)
- Freshly prepared chlorine water bleaches but chlorine water exposed to sunlight for sometime does not bleach. Explain. (2marks)
- When preparing hydrogen chloride gas from sodium chloride and sulphuric (VI) acid, two conditions are necessary. State the conditions. (1mark)
- The reaction between 0.65g of zinc granules and excess of 0.5M hydrochloric acid was followed by measuring the amount of gas produced. The following results were obtained
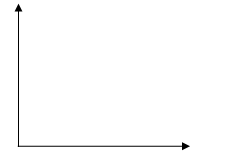
- Plot a graph of volume of gas produced against time. (4marks)
-
- Write an equation for the reaction taking place. (1mark)
- How would the gas produced be identified? (1mark)
- Why is an excess of an acid used? (1mark)
- From the graph:
-
- What is the volume of the gas evolved at 75 seconds? (1mark)
- At what time is the reaction complete? (1mark)
- On the same graph, sketch the curves that you expect if the experiment was repeated under the same conditions but using:
- 0.4M hydrochloric acid instead of 0.5M hydrochloric acid. Label the graph X. (1mark)
- Zinc powder (same quantity) was used in place of granulated zinc. Label the graph Y. (1mark)
- Calculate the volume of the gas that would be produced at r.t.p from 13g of zinc. (Zn = 65.0, molar gas volume at r.t.p. = 24.0dm3) (2marks)
- One mole of Heptane was thermally cracked; two hydrocarbons Q and P were formed. Q was alkene molecule with three carbon atoms.
- Give the name and the structural formulae of:
NAME STRUCTURE
Q (2 marks)
P (2 marks) - Name the compounds that can be used to prepare ethene gas in the laboratory. (1mark)
- An organic compound J has the following percentage composition by mass, carbon, 64.86%, hydrogen, 13.51% and the rest oxygen. The relative molecular mass of the compound is 74. [C=12. H=1 O=16]
- Work out the molecular formula of compound J. (3marks)
- To which homologous series does compound J belong? (1mark)
- Give the name and the structural formulae of:
-
- State two factors that should be considered when choosing fuel for cooking (2marks)
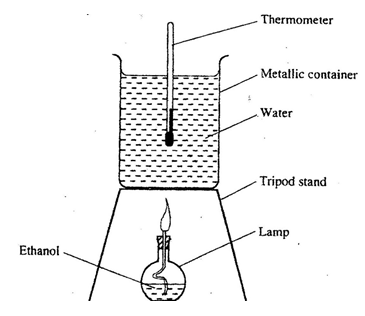
- The diagram below represents a set – up that was used to determine the molar heat of combustion of ethanol(CH3CH2OH).
During the experiment, the data given below was recorded
Volume of water =450cm3
Initial temperature of water =250 C
Final temperature of water =46.50C
Mass of ethanol + Lamp before burning =125.5g
Mass of ethanol + lamp after burning =124.0g
Calculate the:- Heat evolved during the experiment (density of water = 1g/cm3
Specific heat capacity of water = 4.2 kJg-1K-1 (3 marks) - Molar heat of combustion of ethanol (C = 12.0, O = 16.0, H=1.0)( 2 marks)
- Write the thermochemical equation for the above reaction. ( 1mark)
- Heat evolved during the experiment (density of water = 1g/cm3
- The value of the molar heat of combustion of ethanol obtained in (b) (ii) above is lower than the theoretical value. State two sources of error in the experiment. (2 marks)
- On the axes below draw an energy level diagram to represent the above reaction. (2 marks)
- In order to find the proportion by volume of one of the main constituents of air, a sample of air was passed through two wash bottles; the first containing aqueous sodium hydroxide and the second containing concentrated Sulphuric (VI) acid and was then collected in a gas syringe.
- Suggest a reason for passing the air through
- Aqueous sodium hydroxide. (1mark)
- Concentrated sulphuric (VI) acid (1mark)
- The volume of gas collected in the syringe was 80 cm2. This was passed several times over hot copper powder until no further contraction of volume took place. After cooling to the original temperature the volume was found to have reduced to 63.2 cm3.
- How would the copper change in appearance? (1mark)
- Which gas had been removed by the copper? (1mark)
- Calculate the volume of this gas present in the sample. (1mark)
- Calculate the percentage of this gas present in the sample of air. ( 1mark)
- Name the main gas remaining in the syringe. ( 1 mark)
- Suggest a reason for passing the air through
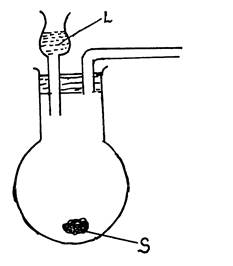
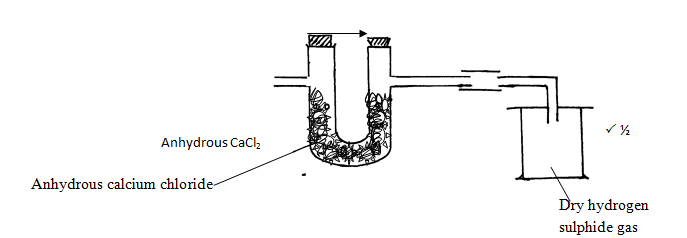
- The set up below is used to prepare and collect dry samples of hydrogen sulphide gas.
- Name suitable substances for use as: (2marks)
L
S - Complete the diagram to show how dry hydrogen sulphide gas is obtained and collecte (2 marks)
- Write a balanced equation for the reaction between L and S named in (a) above. (1mark)
-
- State the effect of hydrogen sulphide gas on litmus. (1mark)
- What do you observe when hydrogen sulphide gas is passed through aqueous zinc chloride? (1mark)
-
- Name the process used to extract sulphur from the ground. (1 mark)
- State the uses of the following materials during extraction of sulphur.
I - Super heated water. (1 mark)
II - Hot compressed air. (1 mark)
-
- Name the process used to manufacture sulphuric acid. (1 mark)
- Calculate the mass of sulphuric acid required to react with excess ammonia gas to produce 125.2 tons of ammonium sulphate fertilizer. (3mks)
- Name suitable substances for use as: (2marks)

MARKING SCHEME
-
- B√ ½ and E ½
- D√ ½
- F √ ½ has smallest atomic radius hence electrons are strongly attracted by positive nucleus √ ½
- D and G //J and G//D and C//K and G (Any one above award 1mk for both
- B and C //I and C//E and C (Any one above award 1 mk for both)
- 1 mk for showing all charges
1 mk for drawing and labeling the atoms - Number of neutrons is not equal to the number of protons.
- K has stronger metallic bonds √ 1 since it has smaller atomic size √ l hence more energy is needed to break them.
- Add water to the mixture and stir √ ½
Sulphate of K dissolves while lead (II) sulphate does not √ ½
Filter √ ½ , lead (II) sulphate as the residue while sulphate of K is the filtrate √ ½
Evaporate the filtrate to dryness √ ½ to obtain solid sulphate of K√ ½
-
- Carbon (iv) oxide, sulphur (iv) oxide, dust particles Any two 1 mk each
- To increase surface area√1
- Reduces wastage √1
- Temperature of 450 – 5000 C
- - As a fertilizer
- Manufacture of nitrogenous fertilizers
- Softening water
- Manufacture nitric (v) acid
- Remove stains in laundry
- Manufacture of hydrazine used as rocket fuel -
- Platinum – rhodium /Platinum
- 4NH3(g) +5O3(g) → 4NO(g) + 6 H2O(g)
- NO reacts with oxygen to form NO2 which dissolves in moisture forming HNO3 which falls as acid rain.// NO3- in water bodies encourages rapid growth of algae leading in a reduction in oxygen content in water. This causes death of aquatic life.
-
- Heat is not required P1
- There would be no change in both red and blue litmus papers. Dry chlorine does not have acidic property and does not bleach.
- Freshly prepared chlorine water has chloric (i) acid) and therefore bleaches. But when exposed to sunlight chloric (i) acid decomposes into hydrochloric acid and oxygen gas is released
- - heat
- the acid must be concentrated
-
- S1
P1
C1 -
- Zn(s) + 2HCl(aq) →Zncl2(aq) + H2(g)
- Extinguishes a burning splint with a ‘pop’ soundP1
- To ensure that all the zinc granules reactedP1
-
- Correct value read from graphP1
- At 180th minuteP1
-
- To the right of the curve in (a) P1
- To the left of the curve in (a) P1
- Zn(s) + 2HCl (aq) →Zncl2(aq) + H2(q) ü ½
1 1
65 g of zinc product 24 litres
\ 13g ” ” x
X=(13 X 24)/65
= 4.8 liters
- S1
- Q C3H6// CH2CHCH3 P1
P C4H10//CH3CH2 CH2CH3 P1-
- Ethanol P ½ and Conc. Sulphuric P ½ acid 1 mk acc. Correct formula of the compounds
- Carbon hydrogen Oxygen
% 64.86 13.51 100 – 78.37 = 21.63
RAM 12 1 16
Moles = 5.405 = 13.51 = 1.352
Mole ratio = 4 = 10 = 1
Empirical formula C4H10O
Molecular formula (C4H10O)n
N = = 1
Molecular formula = C4H10OP ½
- Alcohols / Alkanols P 1
-
-
- - Heating value
- Availability
- Cost
- Ease of storage
- Ease of transporting -
- Heat produced = MC∆T
Mass of water = 100 x 1 = 100g
∆T = 46.5 = 25 = 21.50C
∆H = x 450 x 21.5
=40.635kJ - Molar heat = = 1246.47kj/mol
- Heat produced = MC∆T
- C2H5OH(l) + 3O2(g) 2CO2(g) + 3H2O(l) ∆H = 1246.72kJ/mol
- - Heat lost to the surrounding
- Heat absorbed by the apparatus
- Experimental errors when reading thermometer
- - Heating value
-
-
- To remove carbon (iv) oxide
- To dry the air/remove water vapour
-
- Colour changes from red brown to black
- 80 – 63.2 = 16.8 cm3
- Nitrogen
-
-
- L - Dilute hydrochloric acidP ½
S - Iron (II) SulphideP ½ -
- Fes(s) + 2HCl(aq) →FeCl2(aq) + H2S(g)
-
- H2S turns wet blue litmus paper red P ½ but has
No effect on red litmus. P 1 - A white precipitate is formedP 1
- H2S turns wet blue litmus paper red P ½ but has
-
- Frasch process
- Super heated – To melt P ½ the sulphur deposits
- To force a froath of molten sulphur and water to the surfaceP ½
- 2NH3(g) + H2SO4(aq)→(NH4)2SO4(aq) P ½
98g 132g
132 (NH4)2SO4 º 98
125.2 º
= 95.95tons
- L - Dilute hydrochloric acidP ½
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download CHEMISTRY PAPER 2 - KCSE 2019 MOCK EXAMINATION - KAKAMEGA.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students