Questions
-
-
- Draw the apparatus used in the laboratory for keeping substances free from moisture. (1 mark)
- Name the apparatus used in the laboratory for supporting crucibles on tripod stand while heating. ( 1 mark)
- State two roles chemistry play in the society. ( 1 mark)
-
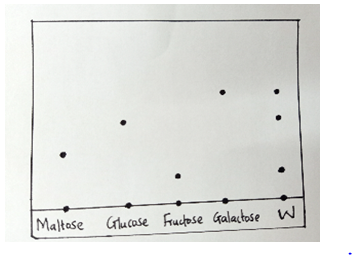
- A sugar called raffinose was treated with dilute hydrochloric acid. The resulting solution W was analyzed to find out the sugars present using chromatography. The following chromatogram was obtained.
- Identify the sugars present in W. ( 1 ½ marks)
- Which of the sugars is less sticky? Explain. ( 1 ½ marks)
-
- Describe how simple acid-base indicators can be obtained from flower petals. . (2marks)
- What is the disadvantage of using phenolphthalein indicator over other commercial indicators. ( 1 mark)
- State and explain two observations made when a burning magnesium ribbon is lowered into a gas jar full of carbon (IV) oxide. ( 3 marks)
- Draw a well labeled set-up of apparatus that can be used in the laboratory preparation of oxygen gas in the laboratory using sodium peroxide and water.( 3 marks)
- Potassium consists of three isotopes with mass numbers Y, 40 and 41 having relative abundances 93.1%, 0.01% and 6.89% respectively. Determine the value of Y given the atomic number of potassium is 19 and its relative atomic mass is 39.1379 . ( 2 marks)
-
- Define the term ionization energy. ( 1 mark)
- Using crosses (x ) to represent electrons, illustrate ion formation by an oxygen atom. ( 2 marks)
- In an experiment to electrolyse a nitrate of element X in solution state using inert electrodes, 386 C of charge produced by passing a current of 0.2A increased the mass of the cathode by 0.128g. If the relative atomic mass of X is 64, determine the oxidation state in an ion of X. ( 1F= 96500C ) ( 2 marks)
- 3 ZnO (s) + 2 NH3(g) → 3 Zn (s) + N2(g) + 3 H2O (l)
In the equation above, identify with a reason the oxidizing agent and the reducing agent. ( 2 marks)
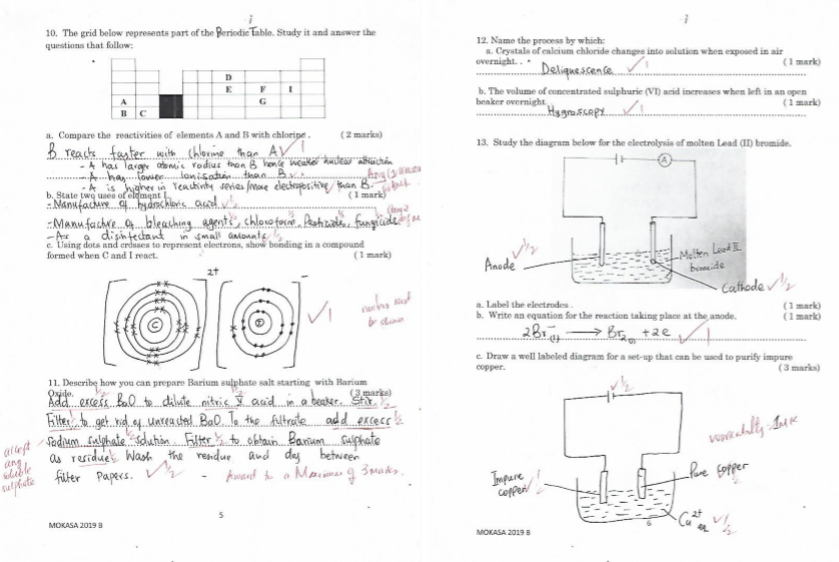
- The grid below represents part of the periodic table. Study it and answer the questions that follow:
- Compare the reactivities of elements A and B with chlorine . ( 2 marks)
- State two uses of element I. ( 1 mark)
- Using dots and crosses to represent electrons, show bonding in a compound formed when C and I react. ( 1 mark)
- Describe how you can prepare Barium sulphate salt starting with Barium Oxide. ( 3 marks)
- Name the process by which:
- Crystals of calcium chloride changes into solution when exposed in air overnight. . ( 1 mark)
- The volume of concentrated sulphuric (VI) acid increases when left in an open beaker overnight. . ( 1 mark)
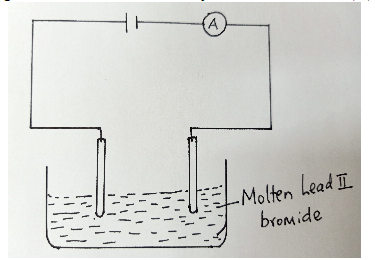
- Study the diagram below for the electrolysis of molten Lead (II) bromide.
- Label the electrodes . ( 1 mark)
- Write an equation for the reaction taking place at the anode. ( 1 mark)
- Draw a well labeled diagram for a set-up that can be used to purify impure copper. ( 3 marks)
-
- What do you understand by the term greenhouse effect? ( 1 mark)
- State two ways in which carbon (IV) oxide is released into the environment( 1 mark)
- Study the diagram below and use it to answer the questions that follow.
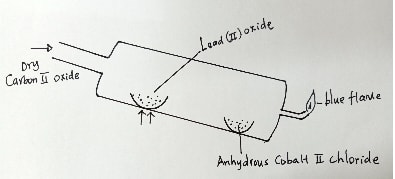
- State what is observed on the anhydrous cobalt (II) chloride. ( 1 mark)
- Write an equation for the reaction taking place in the combustion tube. ( 1 mark)
- State one industrial use of carbon (II) oxide. \ ( 1 mark)
-
- State Graham’s Law of diffusion. ( 1 mark)
- 200 cm3 of oxygen gas took 90 seconds to diffuse through a porous plug. Determine the time taken by 300 cm3 of Sulphur (IV) oxide to diffuse through the same plug under the same conditions. ( O= 16, S = 32 ) . ( 3 marks)
- Copper (II) nitrate was heated carefully and the gases produced passed over water. A colourless gas is collected over water.
- Write an equation to show its decomposition. ( 1 mark)
- If 9.4g of the solid was heated, determine the volume of the gas collected at R.T.P. (3 marks)( Cu= 64, N=14, O= 16 , molar gas volume at R.T.P = 24 dm3)
- Draw the structural formula of the following:
-
- Ethyne ( 1 mark)
- Ethane ( 1 mark)
- Describe an experiment to distinguish between the compounds in (a) above. . ( 2 marks)
-
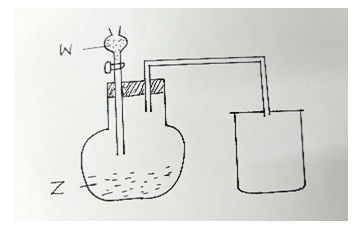
- Study the diagram below for the preparation and collection of nitrogen (IV) oxide in the laboratory and use it to answer the questions that follow.
- Name substances W and Z. ( 2 marks)
W………………………………………………
Z…………………………………………………. - Give a reason why the gas is collected as shown. ( 1 mark)
- State and explain the observations made when moist blue litmus paper is dropped into a jar containing chlorine gas. ( 2 marks)
-
- Given the reaction;
NH4+ (aq) + OH-(aq) NH3 (g) + H2O (l)
Identify the acid in the backward reaction and give a reason for your answer. (2 marks) - State two advantages of hard water. ( 1 mark)
- Given the reaction;
-
- Define the term solubility. ( 1 mark)
- Use the information below to calculate the solubility of sodium nitrate.
Mass of evaporating dish = 15.10g
Mass of evaporating dish and salt = 20.10g
Mass of evaporating dish and solution = 40.10g ( 2 marks)
- 100 cm3 of 2M copper (II) sulphate at 20 0C is reacted with 3 grams of magnesium ribbon. The temperature of the resulting solution was found to be 26 0C. ( density of solution is 1 g/cm3, c= 4.2kJ/Kg/K, Mg=24 )
- Determine the molar enthalpy change for the reaction. ( 3 marks)
- Draw an energy level diagram for representing the reaction (2 marks)
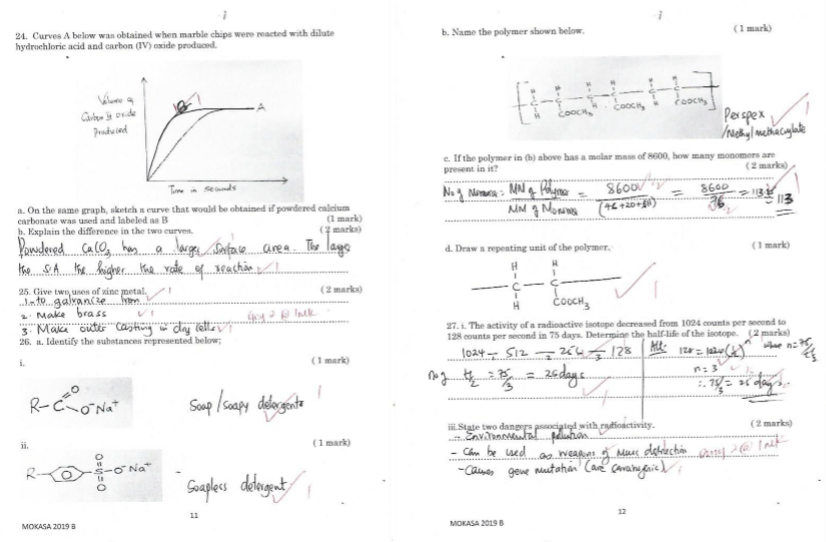
- Curves A below was obtained when marble chips were reacted with dilute hydrochloric acid and carbon (IV) oxide produced.
- On the same graph, sketch a curve that would be obtained if powdered calcium carbonate was used and labeled as B (1 mark)
- Explain the difference in the two curves. ( 2 marks)
- Give two uses of zinc metal. ( 2 marks)
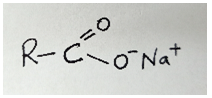
- Identify the substances represented below;
-
( 1 mark)
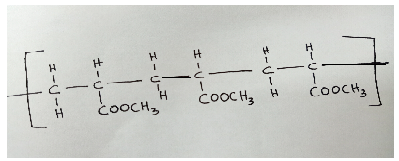
- Name the polymer shown below. ( 1 mark)
- If the polymer in (b) above has a molar mass of 8600, how many monomers are present in it? ( 2 marks)
- Draw a repeating unit of the polymer. ( 1 mark)
-
- The activity of a radioactive isotope decreased from 1024 counts per second to 128 counts per second in 75 days. Determine the half-life of the isotope. ( 2 marks)
- State two dangers associated with radioactivity. ( 2 marks)

MARKING SCHEME
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download CHEMISTRY PAPER 1 - 2019 MOKASA II MOCK EXAMINATION.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students