Instructions to candidates
- Write your name and index number in the spaces above.
- Sign and write the date of examination in the spaces provided above.
- Answer all the questions in the spaces provided in the question paper.
- KNEC mathematical tables and silent non-programmable electronic calculators may be used.
- All working must be clearly shown where necessary.
- This paper consists of 14 printed pages.
- Candidates should check the question paper to ascertain that all the pages are printed as indicated and that no questions are missing.
- Candidates should answer the questions in English
For examiners use only
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | Total |
| 17 | 18 | 19 | 20 | 21 | 22 | 23 | TOTAL |

QUESTIONS
1.
- Identify and state the use of the apparatus represented below. (1 mk)
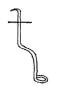
Name:......
Use .......... - State three methods that can be used to separate a solute from a solvent. (11/2 mks)
- ...............
- ...............
- ...............
- Give three factors that help in separation of mixture by chromatography. (11/2mks)
- ...............
- ...............
- ...............
2.
- State the kinetic theory of matter. (1 mk)
- Using kinetic theory, explain the fact that the volume of a fixed mass of a gas is inversely proportional to its pressure at constant temperature. (2 mks)
- Why is it necessary to apply heat in order to change from one state of matter to another? (1 mk)
3. Name the homologous series to which the functional groups below belong. (2 mks)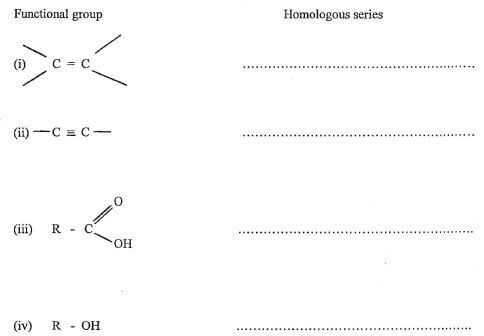
(b) Complete the following equations. (11/2 mks)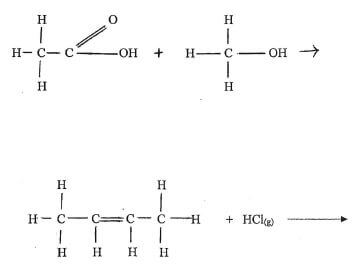
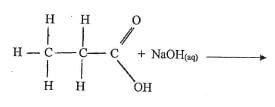
(c) Name the type of reaction represented by each of the equations completed in part (6) above. (11/2 mks)
- .............
- .............
- .............
4. A form two student wanted to carry out an experiment to observe which liquids can allow passage of electric current through them.
- Using water as one of the liquids used, draw a well labelled set up that the student used for the experiment. (3 mks)
- What observation did she make in her set up for:
- A liquid that allowed passage of an electric current? (1/2 mk)
- Aliquid that didn't allow passage of electric current (1/2mk)
5. (a) Hydrogen sulphide gas is bubbled through bromine water.
- State the two observations made
- ........
- ........ (1 mk)
- Write an equation for the reaction that takes place. (1 mk)
(b) What are the chemical and physical identification tests for hydrogen sulphide? (2 mks)
Physical .......
Chemical .......
6. A form three student was required to carry out a titration practical to help determine the
concentration of a given sodium hydroxide solution labeled solution 'A' She was provided with
- IM hydrochloric acid labeled solution 'B'
- Phenolpthalein indicator
- Necessary apparatus
Write a simple procedure in four steps that the student should follow to carry out the experiment. (4 mks)
7.
- Oxygen gas can be prepared in the lab. by catalytic decomposition of hydrogen peroxide. Name two other chemicals that can be heated to produce oxygen in the lab.(2 mks)
- Air is said to be a mixture and not a compound. State two reasons to justify this. (2 mks)
8.
- Differentiate between electronegativity and electron affinity (1 mk)
- State and explain the difference in electron affinity in chlorine and argon (2 mks)
(atomic number Cl=17 Ar-18)
9.
- In terms of structure and bonding, explain the fact that melting point of group one elements decreases down the group. (2 mks)
- Draw a dot and cross diagram to show bonding in ammonia molecule. (1 mk)
(atomic number N-7, H=1) - An atom is made of three subatonic particles i.e neutron, protons and electrons. Using these particles, explain the meaning of nuclear force of attraction (1 mk)
10.
- Write down thermochemical equations to represent the following heat changes.
- Standard molar heat of formation of water given its value as △HRØ=-571.6kJ/mol (1 mk)
- Standard molar heat of combustion of methane given its value as △HØ = -890.3kJ/mol. (1 mk)
- The diagram below shows an energy cycle.
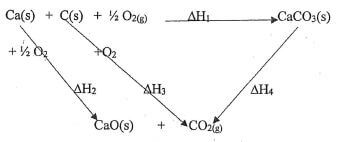
Given △H1 = -1207Kjmol-1
△H2 = -635Kjmol-1
△H3 = -394Kjmol-1
Determine the value of △H4. Show your working. (2 mks)
11.
- Give one reason some of the laboratory apparatus are made of ceramics. (1 mk)
- Name two apparatus that can be used to measure approximately 75 cm' of dilute sulphuric (VI) acid. (2 mks)
12. A tea farmer suspected that her farm had turned acidic. She obtained a soil sample to analyze for pH. Give her the procedure to follow in order to verify this. (2 mks)
13. When magnesium is reacted with steam, it reacts rapidly forming a white solid and hydrogen gas.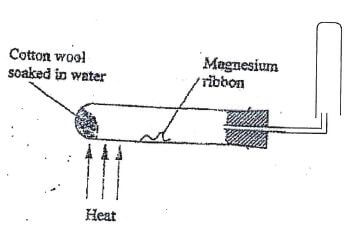
- What property of hydrogen gas makes it to be collected as shown above. (I mk)
- How would you show that the gas collected is hydrogen gas? (Imk)
- When copper turnings were used instead of magnesium ribbon, hydrogen gas was not produced. Explain. (Imk)
14. Describe how to prepare crystals of copper(II) sulphate starting with copper turnings. (3 mks)
15. Study the table below and answer the questions that follow.
| Element | Atomic radii (nm) | Ionic radii (nm) |
| Flourine | 0.071 | 0.136 |
| Chlorine | 0.099 | 0.181 |
| Bromine | 0.114 | 0.195 |
- Explain why
- Atomic radius increases from fluorine to bromine (2 mks)
- The ionic radius is larger than the atomic radius. (2 mks)
16. The diagram below represents a setup used by a student to investigate the effect of heat on sodium nitrate. Use it to answer the questions that follow. 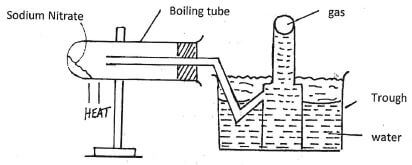
a) Write the chemical equation of the reaction in the boiling tube. (Imk)
b) State the property of the gas that makes it to be collected by the method shown. (1 mk)
c) Predict the effect of water in the trough on litmus paper after the experiment (Imk)
17. The set-up below was used to prepare dry chlorine gas. Study and answer the questions that follow.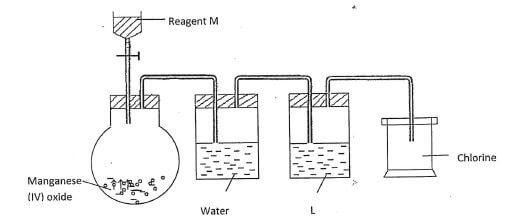
- Name reagents M and substance L.
M:...................................................................(1/2 mk)
L: ...................................................................(1/2 mk) - A warm red phosphorus was lowered into the gas jar of chlorine using a deflagrating spoon:
- State any one observation made in this experiment (1/2 mk)
- Identify the substance formed in the above reaction.(1/2 mk)
- Both substances in (ii) above undergo hydrolysis when exposed to air. Write an equation to show how anyone of them undergoes hydrolysis. (1 mk)
18.During the manufacture of sodium carbonate in the industry.
- Give the name of the process used to manufacture sodium carbonate. (Imk)
- Write the final equation for the formation of sodium carbonate during the process. (Imk)
- Give one use of sodium carbonate. (Imk)
19.
- Nitrogen (1) Oxide supports combustion of burning charcoal. Write an equation to show this reaction (1mk)
- Ammonium nitrate can be heated to give off Nitrogen (L) oxide. However a mixture of NH4Cl and NaNO3 is preferred. Explain (Imk)
- State the physical test of Nitrogen (1) Oxide (Imk)
20. Archaeologists can determine the age of organic matter by measuring the proportion of carbon -14 present in a sample. Assuming that carbon- 14 has a half-life of 5600 years, calculate the age of a piece of wood found to contain 1/8 as much carbon - 14 as in a living material (3mks)
21. A saturated solution of sodium nitrate in water was made at 30°C. Use the information below to answer the questions that follow.
Mass of evaporating dish = 52.5g
Mass of evaporating dish + salt solution = 119.6g
Mass of evaporating dish + dry salt = 59.3g
- What is solubility? (1 mk)
- Determine the solubility of sodium nitrate at 30°C. (2 mks)
22. Study the equilibrium reaction below and answer the questions that follow.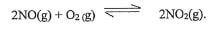
The forward reaction is exothermic. How would the following affect the position of the equilibrium?
- The temperature of the system is lowered. Explain. (11/2mk)
- The pressure of the system is lowered. Explain. (11/2mk)
23. The diagram below represents the second stage in extraction of aluminium metal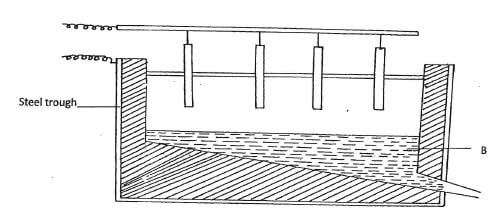
- Write the formula of bauxite (Imk)
- How is the ore (bauxite) concentrated before it is electrolyzed (Imk)
- What is the purpose of dissolving electrolyte B in molten cryolite (Na3AlF6)
- (Imk)
Download Chemistry Paper 1 Questions - Alliance Girl's High School Mock December 2020.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

