QUESTIONS
- You are provided with:
- Solution A, containing 4.0gdm-3 of sodium hydroxide
- Solution B, hydrochloric acid
- 2.5 g of a mixture of two salts, XCl (RFM 58.5) and X2CO3 (RFM 106)
You are required to:
- Standardize solution B, hydrochloric acid.
- Determine the mass composition of the salt mixture
PROCEDURE I
- Fill the burette with solution B
- Pipette 25 cm3 of solution A into a clean dry conical flask. Then add 2 -3 drops of phenolphthalein indicator.
- Titrate solution A solution with solution B. Record your results in the table below.
- Repeat the procedure two more times to retain concord and values.
TABLE 1 (3 marks)
| Titration number | 1 | 2 | 3 |
| Final burette reading (cm3) | |||
| Initial burette reading(cm3) | |||
| Volume of acid used (cm3) |
- Calculate the average volume of solution B used. (1mark)
- Find;
- Moles of sodium hydroxide that reacted with the acid. (2 marks)
- Moles of hydrochloric acid present in the average volume. (1mark)
- Molarity of the acid (1mark)
PROCEDURE II
- Put about 100cm3 of water in a 250ml volumetric flask add all the 2.5g of salt mixture. Shake the mixture to dissolve and the solid. Top up the solution to the mark with distilled water Label this solution C
- Fill this burette with solution B.
- Pipette 25c m3 of solution C and put it into a clean conical flask. Add 3 drops of methyl orange indicator.
- Titrate solution C with solution B. Record your results in the table below.
- Repeat the titration two more times
TABLE II (3 marks)
| Titration number | 1 | 2 | 3 |
| Final burette reading (cm3) | |||
| Initial burette reading(cm3) | |||
| Volume of acid used (cm3) |
- Calculate the average volume of solution B (1mark)
- Calculate the number of moles in the hydrochloric acid used (1mark)
- The equation for the reaction of the acid with one of the salts in the mixture is:
2HCl(aq) + X2CO3(s) → 2XCl(aq) + CO2(g) + H2O(l)
Calculate;- Moles of X2CO3 that reacted with the acid in the experiment (1mark)
- Molarity of X2CO3 (2 marks)
- Calculate the mass of the salt mixture in gdm-3. (1mark)
- Calculate the percentage of XCl in this mixture (2 marks)
- In this experiment, you’re required to determine the time takes for a precipitate to be formed when S3 which is sodium thiosulphate solution, reacts with dilute hydrochloric acid.
PROCEDURE
- Using a measuring cylinder measure 50cm3 of S3 into a 100ml beaker.
- Make a pencil cross on a white piece of paper so that when a beaker is placed top of the paper , the cross can be seen through the bottom of the beaker.
- To solution A add 10 cm3 of 2M hydrochloric acid and at the same time start a stop watch / stop clock. Swirl the contents of the beaker twice and then place it over the cross on the paper. Look at the cross from above the beaker through the mixture. Stop the stop watch immediately the precipitate makes the cross invisible. Record time taken for the cross to become invisible in the table below, rinse beaker.
- Repeat the procedure with solutions B, C, D and E as per the table.
SOLUTION Volume of solution S3 in the beaker (cm3) Volume of water added (cm3) Volume of 2M HCl Time taken in seconds A 50 0 10 B 40 10 10 C 30 20 10 D 20 30 10 E 10 40 10 - Plot the graph of volume of solution S3 (y – axis) against time (4 marks)
- From the graph state the relationship between concentration of solution S3 and time. (1mark)
- Why is water added to the solution S3? (1mark)
- You’re provided with solid D. Carry out the tests shown below on the solid.
- Heat a spatula full of D in A clean dry test – tube.
OBSERVATIONS INFERENCES - Put a spatula end- full of D in a boiling tube. Half fill it with water. shake this mixture
OBSERVATIONS INFERENCES - Divide the resultant mixture in (b) above into 5 portions
OBSERVATIONS INFERENCES - To the first portion add dilute nitric acid followed by a few drops of Barium nitrate
OBSERVATIONS INFERENCES - To the second portion, add nitric acid a few drops followed by lead (II) nitrate and then warm the mixture.
OBSERVATIONS INFERENCES - To the third portion, add sodium hydroxide solution drop wise until in excess. Warm this mixture. Test any gas produced withy Litmus paper
OBSERVATIONS INFERENCES
- To the first portion add dilute nitric acid followed by a few drops of Barium nitrate
- You are provided with liquid B. Carry out the tests shown below and write your observations and inferences in the spaces provided.
- To about 1cm3 of liquid B in a test – tube, add about 1cm3 of distilled water and shake the mixture.
OBSERVATIONS INFERENCES - To about 1cm3 of liquid B in a test tube add a small amount of solid sodium hydrogen carbonate
OBSERVATIONS INFERENCES - To about 2cm3 of liquid B in A test – tube, add about 1cm3 of acidified potassium dichromate (VI). Warm the mixture gently and allow it to stand for about one minute.
OBSERVATIONS INFERENCES
- To about 1cm3 of liquid B in a test – tube, add about 1cm3 of distilled water and shake the mixture.
- Heat a spatula full of D in A clean dry test – tube.
CONFIDENTIAL
Each student requires
- 80 cm3 of solution A prepared by dissolving 4.0g of NaOH in water made up to 1 litre.
- 200cm3 of solution B which is 0.1M HCl.
- 2.5 of a salt mixture prepared by mixing 1.5 g of sodium carbonate (Anhydrous) and 1.0g of sodium chloride.
- One burette (50ml)
- One 25cm3 pipette
- Pipette filler
- Complete stand
- Filter funnel
- White tile
- 3 conical flasks
- 250ml volumetric flask.
- Labels (6)
- 500ml distilled water in a wash bottle.
- 200cm3 of solution S3 which is sodium thiosulphate of concentration 0.2 M
- 80cm of 2m hydrochloric acid solution
- 100ml empty glass beaker.
- One stop watch/ clock.
- white piece paper
- One 50ml measuring cylinder
- About 2g of solid D (A mixture of ammonium sulphate and zinc sulphate in the ratio 1:1).
- About 5cm3 of liquid B which is absolute ethanol
- Two red litmus paper.
- One metallic spatula.
- About 1g of solid sodium hydrogen carbonate
- Test- tube holder.
- One boiling tube
- Seven clean dry test tubes.
Provide access to:
- Means of heating
- Phenolphthalein indicator
- Methyl - indicator
- Acidified potassium dichromate (vi) solution.
- 2m nitric (V) acid solution
- 2m Barium nitrate solution
- 2m ammonia solution.
- 2m sodium hydroxide solution.
- 2m lead nitrate solution
Note: The solution should be supplied with droppers.

MARKING SCHEME
Table 1
| Titration number | 1 | 2 | 3 |
| Final burette reading (cm3) | 24.5 | 24.5 | 24.5 |
| Initial burette reading(cm3) | 0 | 0 | 0 |
| Volume of acid used (cm3) | 24.5 | 24.5 | 24.5 |
Marking
- Complete table award;
- Decimal consistency;
- Accuracy 0.1;
- School value;
Principles of averaging:
Average volume = 24.5 + 24.5 + 24.5 = 24.5; (½ mark)
3
= 24.5 cm3;(½ mark)
-
- Moles of sodium hydroxide used
Molarity of solution:
Moles = Mass /litre
RMM
= 4/40 = 0.1molar
If 1000 cm3 → 0.1mole
Then 25 cm3 → 25 x 0.1 = 0.0025 moles;
1000 - Moles of hydrochloric acid
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
Mole ratio = 1:1;
Thus moles of acid = 0.0025 moles; - Molarity of acid.
Volume of acid reacting = average titre in (a) e.g. 24.5 cm3
If 24.5 cm3 → 0.0025 moles
Then 1000 cm3 → 1000 x 0.0025 = 0.1020 molar;
24.5
- Moles of sodium hydroxide used
- Table II
Marking:
Complete table - 1mark
Decimal consistency - 1 mark
Accuracy - 1mark
School value - 1mark
Expected titre = 28.3 cm3 - Average volume
28.3 + 28.3 + 28.3 = 28.3 cm3
3
All 3 values within 0.1 of each other and used – 1mark
Only 2 within 0.1 of each other and used – ½ mark
Inconsistent value used – 0 mark - Answer in (b) (iii) x average volume
1000
Example:
Molarity of the acid calculated in (b) (iii) = 0.102 molar.
Thus 1000 cm3 → 0.102 moles
28.3 cm3 → 28.3 x 0.102 = 0.0028866 moles;
1000 -
- Moles of carbonate that reacted:
Using mole ratio, 2 moles of acid reacts with 1 mole of carbonate
Thus moles of carbonate reacting = answer in (d) x 1
2
Example:
2 moles of acid → 1 mole of carbonate
Thus 0.0028866 moles of acid → 0.0028866 x 1
2
= 0.0014433 moles; - Molarity of carbonate
25 cm3 of carbonate → 0.0014433
Then 1000 cm3 → 1000 x 0.001443 = 0.057732 molar
25
- Moles of carbonate that reacted:
- Mass of the salt mixture in gdm-3
250 cm3 → 2.5g
1000 cm3 → 1000 x 2.5 = 10g;
250 - Percentage of XCl in the mixture
Mass of X2CO3 in 1 litre
Molarity = 0.057732 molar / answer in (e) (ii).
Mass = 0.057732 x 106 = 6.119592g;
Mass of XCl = 10 – 6.119592
= 3.880408;
Percentage = 3.880408 x 100
10
= 30.80408%
Note: Use school based values.
Question 2
-
SOLUTION Volume of solution S3 in the beaker (cm3) Volume of water added (cm3) Volume of 2M HCl Time taken in seconds A 50 0 10 20 B 40 10 10 25 C 30 20 10 35 D 20 30 10 53 E 10 40 10 103 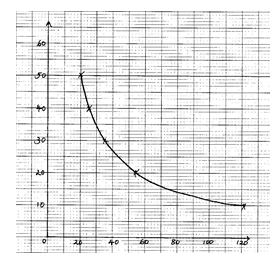
Marking:- Complete table
- Decimal consistency
- Trend (increasing time)
- School value
Graph Trend:
Marking:
Scale – ½ mark
Axes – ½ mark
Plotting – 1mark
Curve – 1mark -
As the concentration decreases ,the time increases
-
To keep the column of solution constant through the experiment
Question 3
| Observations | Inferences |
| (a) Colourless gas with a pungent smell; Gas changes moist red litmus paper blue, moist blue litmus paper remains blue |
NH4+ present; |
| (b) Dissolves to form a colourless solution; | Soluble salt present; Coloured ions absent // Fe2+, Fe3+, Cu2+ absent; |
| (c) (i) White precipitate formed | SO42- present; |
| (ii) White precipitate that persists on warming; | Cl- absent; |
| (iii) - White precipitate that dissolves in excess; - Colourless gas with a pungent smell on warming; Colourless gas changes moist red litmus paper to blue, blue litmus paper remains blue; |
Zn2+ NH4+ present; |
| (e) (i) Dissolves to forma colourless solution |
Polar substance; R – OH present; |
| (ii) No effervescence |
R – COOH, H+, H3O+ absent; |
| (iii) Colour of acidified potassium dichromate (VI) changes from orange to green; | R – OH present; |
Download Chemistry Paper 3 Questions and Answers - Asumbi Girls Mock Examinations 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
