INSTRUCTIONS TO CANDIDATES
- Answer all the questions.
- Mathematical tables and non-programmable electronic calculators may be used.
- ALL working must be clearly shown where necessary.
- Study the information in the table below and answer the questions that follow. The letters do not represent the actual symbols of the elements.
Elements Electronic configuration Ionization energy kjmol−1 P 2:1 519 C 2:8:1 494 R 2:8:8:1 418 - What is the general name given to the group which elements P, C and R belong? (1mk)
- What is meant by ionization energy? (2mks)
- Explain why element P has the highest ionization energy. (2mks)
-
- When a piece of element “C” is placed on water, it melts and hissing sound is produced as it moves on the surface of the water. Explain these observations. (2mks)
- Distinguish between a strong and a weak base. Give an example of each. (2mks)
- Neutralization is one of the methods of preparing salts.
- What is meant by neutralization? (1mk)
- Describe how you would prepare crystals of sodium nitrate starting with 200cm3 of 2M sodium hydroxide. (3mks)
- Write an equation for the reaction that takes place when a solid sample of sodium nitrate is heated. (1mk)
-
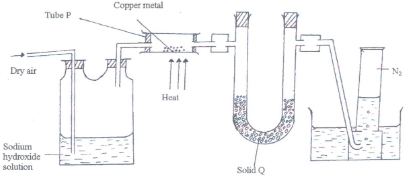
- The diagram below represents a set-up that was used to obtain dry nitrogen from air. Study it and answer the questions that follow.
- Name solid Q. (1mk)
- What is the purpose of NaOH(aq)? (1mk)
- Write an equation for the reaction which took place in tube P. (1mk)
- Give the name of one impurity in the nitrogen gas obtained. (1mk)
- Why is liquid nitrogen used for storage of semen for artificial insemination? (1mk)
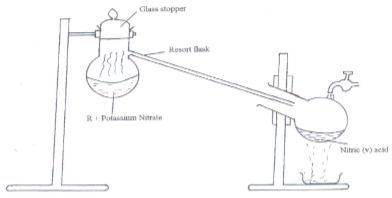
- The set-up below was used to prepare nitric acid.
- Give the name of liquid R. (1mk)
- Write an equation for the reaction which took place in the retort flask. (1mk)
- Explain the following:-
- Nitric acid is not stored in clear / transparent glass. (2mks)
- The reaction between copper metal with 50% nitric acid (one volume of acid added to an equal volume of water) in an open test tube produces brown fumes. (2mks)
- The diagram below represents a set-up that was used to obtain dry nitrogen from air. Study it and answer the questions that follow.
- Chlorine is a member of group VII.
- What is the family name of group VII elements? (1mk)
- Explain why the reactivity of Iodine is lower than that of Bromine. (2mks)
- Chlorine gas used for industrial purposes is obtained by electrolysis of brine.
- Name two other products of this electrolysis. (2mks)
- Write the equation for the reaction at the anode. (1mk)
- Sodium chlorate (NaClO3) is used as a herbicide. It is formed according to the following equation:
6NaOH(aq) + 3Cl2(g) → NaClO3(aq) + 5NaCl(g) + 3H2O(l)
Sodium chloride is less soluble than sodium chlorate and crystallizes out first. Sodium chlorate is obtained from the remaining solution by crystallization of saturated solution.- State one condition necessary for this reaction. (1mk)
- Calculate the maximum mass of sodium chlorate in kilograms that can be formed from 206m3 of chlorine gas. (1 mole of gas = 24dm3, Na = 23.0, Cl = 16.0). (3mks)
- Name two other uses of chlorine. (2mks)
-
- The table below contains information from the measurements made of the radioactivity in counts per minutes from a radioisotope iodine – 128.
Counts per min 240 204 180 156 138 122 108 Time (min) 0 5 10 15 20 25 30 - Plot a graph of counts per minute against time. (3mks)
- Use the graph to determine the half-life of iodine – 128. (1mk)
- What is the counts rate after 22 minutes? (1mk)
- After how many minutes were the counts rate 160 counts per minute? (1mk)
- If isotope
decays to
as a result of X alpha-particles and Y-beta particles
- Find the numerical value of X and Y. (2mks)
- Write the nuclear equation. (1mk)
- A radioactive material emitted radiations as shown below.
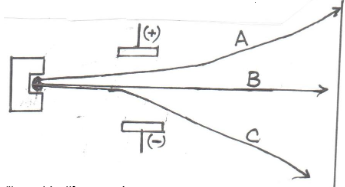
- Identify:
- A (1½mks)
- B
- C
- Which radiation: (1½mks)
- Has no mass?
- Has the lowest ionizing power?
- Contains Helium particles?
- Identify:
- The table below contains information from the measurements made of the radioactivity in counts per minutes from a radioisotope iodine – 128.
- The raw material for extraction of aluminum is bauxite.
- Name the method that is used to extract aluminium from bauxite. (1mk)
- Write the chemical formula for the major components of bauxite. (1mk)
-
- Name the major impurities in bauxite. (2mks)
- Explain how the impurities in bauxite are removed. (1mk)
- Crayolite is used in the extraction of aluminium from bauxite. State its function. (1mk)
- Describe how carbon (IV) oxide is formed during the extraction of aluminium. (2mks)
- Aluminum is a reactive metal yet utensils made from aluminium do not corrode easily. Explain this observation. (2mks)
- Standard electrode potentials for half cell reactions are shown below.
A2+(aq) + 2e → A(s) − 0.76V
B2+(aq) + 2e → B(s) − 0.13V
C+(aq) + e → C(s) +0.80V
D2+(aq) + 2e → D(s) +0.34V
The cell below was set-up using A and B electrodes.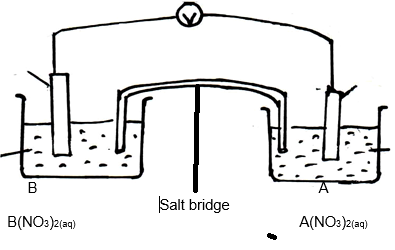
-
- Give the half-cell equations for each half cell. (1mk)
- Write the overall cell equation. (1mk)
- Calculate the e.m.f. of the cell above. (1mk)
- Describe how the salt bridge helps in maintaining the change balance in each half cell when the cell is in operation. (2mks)
- It is not advisable to use potassium chloride salt bridge when Lead (II) nitrate solution is used as an electrolyte in the above set-up. Explain. (2mks)
- Sodium hydroxide is a chemical that can be prepared industrially in a mercury cell.
- Give the name of the main raw material used. (½mk)
- In the cell, graphite is used as anode electrode. Name another substance that can be used in place of graphite (carbon). (½mk)
- Give two uses of sodium hydroxide. (2mks)
- Give two reasons why mercury is recycled. (2mks)
- Write the equation in which sodium hydroxide is produced. (1mk)
- If the volume of hydrogen produced in the mercury cell is 100 litres. Calculate the mass of sodium hydroxide formed at room temperature.
(H = 1.0, Na = 23.0, O = 16.0 MGV = 24 litres) (2mks)
-
-
- Name two advantages of synthetic fibre over natural fibre. (2mks)
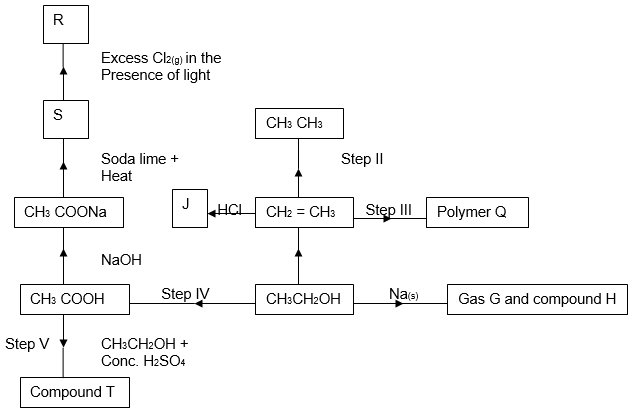
- The scheme below shows reactions starting with ethanol. Study it and answer the questions that follow.
- Name gas G. (1mk)
- Write the structural formulae and names of:
- T (1mk)
- S (1mk)
- Name the homologous series to which T belongs. (1mk)
- Write down the structural formula of compound R. (1mk)
- Name the compound R. (1mk)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions - Mangu High School Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students