- The table below gives the information in four elements elements represented by letters K, L, M and N. Study it and answer the questions that follow.The letters do not represent the actual symbol of the elements.
Element Atomic number Electron arrangement Atomic radius Ionic radius K 2, 8, 2 0.136 0.065 L 17 0.099 0.181 M 2, 8, 8, 1 0.203 0.133 N 20 0.174 0.099 - Complete the table by filling in the missing atomic numbers and electron arrangements (2 mrks)
- Which two elements have similar properties? Explain. (2 mrks)
- What is the formular of the oxide M?
- Which element is non-mental? Explain. (2mrks)
- Which one of the element is the strongest reducing agent? Explain.
- Explain why ionic raduis of N is less than that of M (1 mrk)
- Expalin why ionic radius of L is bigger than it's atomic radius ( 2 mrks)
-
- Define the term molar enthalpy of neutralization (1 mrk)
- In an experiment to determine the molar enthalpy of neutralization 25.0cm3 of 2M sulphuric (iv) acid was added to 50cm3 of 2M sodium hydroxide in a lagged plastic beaker. The mixtutre was stirred with a thermometer and the final temperature attained recorded. The full results obtained in the experiment were as follows:
Volume of 2M sulphuric(iv) acid used 25.0cm3
Initial temperature of the acid T1 19.0°c
Volume of 2M sodium hydroxide used 50.0cm3
Initial temperature of the hydroxide T2 21.0°C
Final temperature attained T4 = 27.5°C
Given that the specific heat capacity of the mixture, C = 4.2KJ/Kg/k and that the desnisty of tghe mixture is 1g/cm3, use the results above to answer the follwoing questions.- Find T3 the common initial temperature (1 mrk)
- Calculate the heat change during the experiment (3 mrks)
- Work out the molar heat of neautralzation (1 mrk)
- Write the thermochemical ionic equation for this process (1 mrk)
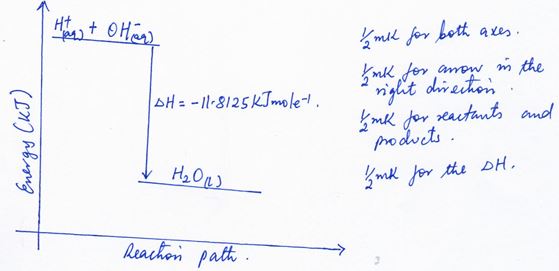
- Draw the energy level diagram for the process (1 mrk)
- State two sources of error in this experiment (2 mrk)
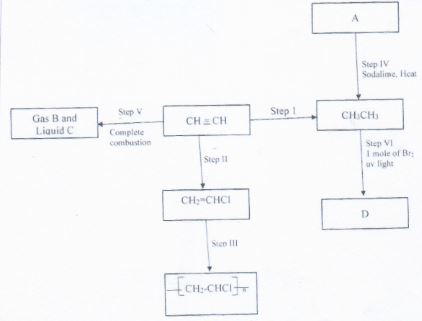
- Study the scheme given above and answer the questions that follow.
- Name the reagents used in :
Step I (½ mrk)
Step II (½ mrk) - Name substances (2 mrks)
- Write an equation for the reaction that takes place in: (3mrks)
Step IV
Step V
Step VI - Expalin one disadvantage of the continued use of items made from the compound formed in step III (1 mrk)
- Name the type of reaction that takes place in
Step I
Step III
Step V
Step VI - State the condition necessary for step II and III to take place. ( 2 mrks)
Step II
Step III
- Name the reagents used in :
-
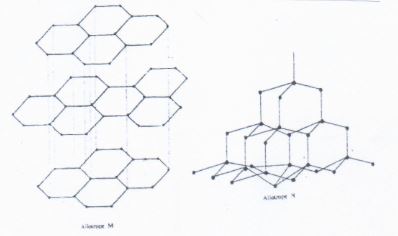
- The following diagrams shows the structure of two allotropes of carbon. Study them and answer the questions that follow:
- Name allotrope (1mrk)
M
N - Give on use of N (1mrk)
- Which allotrope conducts electricity? Explain in terms of structure and bonding (2mrks)
- Name allotrope (1mrk)
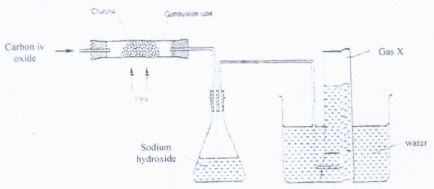
- In an experiment, carbon (iv) oxide gas was passed over heated charcoal and the gas produced collected as show in the diagram that follows;
- Write the equation for the reaction that place in the combustion tube. (1 mrk)
- State the purpose of sodium hydroxide in the set up and explain how it works using a chemical equation. (2 mrk)
- Describe a simple chemical test that can be used to distinguish between carbon (iv) oxide and carbon(ii) oxide (2 mrk)
- Give one use of carbon (ii) oxide (1 mrk)
- The following diagrams shows the structure of two allotropes of carbon. Study them and answer the questions that follow:
- The table below gives the standard reduction potentials of some elements represented by letters U, V, W, X and Z (They are not the actual symbols)
Element Standard electrode potentials (volts) -
- Identify the strongest reducing agent (1 mrk)
- Which two half cells would produce the highest e.m.f (1 mrk)
- Determine th e.m.f obtained from the cell above (2 mrk)
- What would element X represent? (1 mrk)
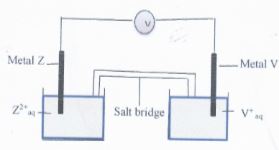
- Element V and Z were connected to form an electrochemical cell as shown in the diagram below
- Write the equation for the reaction that occurs at;
Metal Z elecrode (1 mrk)
Metal V electrode (1 mrk) - Write the cell representation for the above electrochemical cell (1 mrk)
- Determine the e.m.f of the above cell (1 mrk)
- Write the overall cell reaction indicating the e.m.f ((1 mrk)
- State one use of a salt bridge and name two salts that can be used in the salt bridge
Use V (1 mrk)
Salt (1 mrk)
- Write the equation for the reaction that occurs at;
-
-
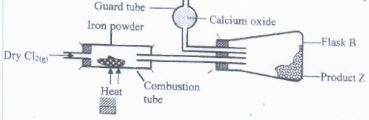
- The set-up below was used to react dry chlorine gas with iron powder. Thwe product Z was collected in flask B.
- Identify product Z
- What property of product Z makes it possible to be collected as shown in the diagram. (1 mrk)
- Expalin why calcium oxide would be preferred to calcium(II) chloride to guard tube. (1 mrk)
- The total mass of product Z formed was found to be 0.5g. Calculate the volume of chlorine gas that reacted with iron ( Fe = 56, Cl = 35.5, MG. V at 298K = 24,0000cm3) (3 mrks)
- Name each processes described below which takes place when salt are exposed to air for sometime.
- Anhydrous copper (II) sulphate becomes wet (1 mrk)
- Common table salt forms an aqueous solution (1 mrk)
- Fresh crystal of sodium carbonate, Na2C03.10H2O
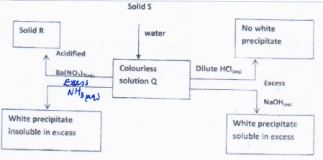
- Study the scheme below and answer the question that follow
Identify solution Q and solid R- Solution Q (1 mrk)
- Solid R (1 mrk)
- Write an ionic equation for the reaction between solution Q and excess aqueous ammonia. (1 mrk)
- The set-up below was used to react dry chlorine gas with iron powder. Thwe product Z was collected in flask B.
-
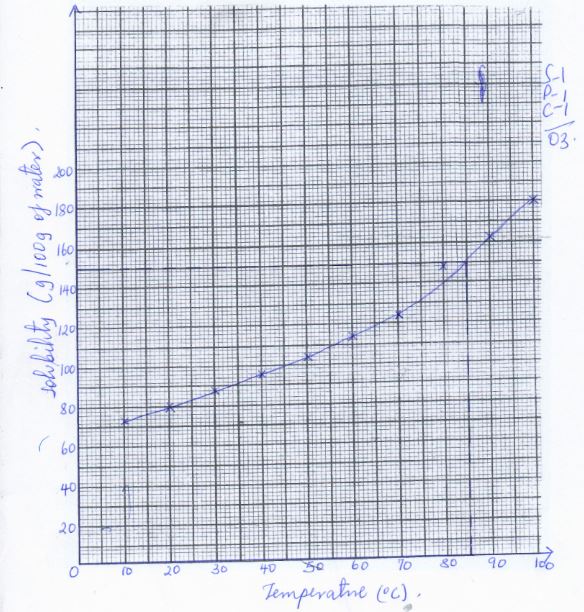
- The solubility in grammes of sodium nitrate in 100g of wterr are given below for various temperatures
Temp (°C) 10 20 30 40 50 60 70 80 90 100 Solubility(g/100g of water) - Plot a graph of solubility of sodium nitrate (y-axis) against temperature (x-axis) (3 mrk)
- Determine the temperature at which the solubility of the salt is 150g/100g of water (1 mrk)
- Given 100g of a saturated solution of sodium nitrate at 100°C, calculate the mass of solute in the solution
- Lead (II) sulphate can be prepared by double decomposition
- What is meant by double decomposition
- Starting with 1.0M sodium sulphate descibe how you would prepare lead (II) sulphate solid. (2 mrk)
- A student analyzed a solution of salt L as follows.
Portion Test Observation I A few drops of dilute hydrochloric acid added to the solution No ppt II A few drops of dilute sulphuric (IV) acid White ppt II A few drops of lead(II) nitrate solution added and then warmed White precipitate which dissolves on warming - Identify the actions that were most probably present in the solution. (1 mrk)
- Write an ionic equation for the observation made in the test III (1 mrk)
- The solubility in grammes of sodium nitrate in 100g of wterr are given below for various temperatures
MARKING SCHEME
- The table below gives the information in four elements elements represented by letters K, L, M and N. Study it and answer the questions that follow.The letters do not represent the actual symbol of the elements.
Element Atomic number Electron arrangement Atomic radius Ionic radius K 12 2, 8, 2 0.136 0.065 L 17 2, 8, 7 0.099 0.181 M 19 2, 8, 8, 1 0.203 0.133 N 20 2, 8, 8, 2 0.174 0.099 - Complete the table by filling in the missing atomic numbers and electron arrangements (2 mrks)
- Which two elements have similar properties? Explain. (2 mrks)
- K and N − They belong to the same chemical family
- What is the formular of the oxide M?
- M2O
- Which element is non-mental? Explain. (2mrks)
- L − Forms ion by gaining one electron
- Which one of the element is the strongest reducing agent? Explain.
- M − Loses electrons most readily/Most elecropositive
- Explain why ionic raduis of N is less than that of M (1 mrk)
- The ion of N has more protons tahn trhat of M and hence experiences stronger nuclear attractive force.
- Expalin why ionic radius of L is bigger than it's atomic radius ( 2 mrks)
- L − Forms ions by gaining an electron. This increases the repulsion between the electrons in the outtermost energy level and reduces the effective nuclear attractive force
-
- Define the term molar enthalpy of neutralization (1 mrk)
- The heat change that occurs when one mole of water is formed through acid-base neutralization reaction
- In an experiment to determine the molar enthalpy of neutralization 25.0cm3 of 2M sulphuric (iv) acid was added to 50cm3 of 2M sodium hydroxide in a lagged plastic beaker. The mixtutre was stirred with a thermometer and the final temperature attained recorded. The full results obtained in the experiment were as follows:
Volume of 2M sulphuric(iv) acid used 25.0cm3
Initial temperature of the acid T1 19.0°c
Volume of 2M sodium hydroxide used 50.0cm3
Initial temperature of the hydroxide T2 21.0°C
Final temperature attained T4 = 27.5°C
Given that the specific heat capacity of the mixture, C = 4.2KJ/Kg/k and that the desnisty of tghe mixture is 1g/cm3, use the results above to answer the follwoing questions.- Find T3 the common initial temperature (1 mrk)
T3 = T1 + T2 = 19.0 + 21.0
2 2
= 40.0
2 = 20.0°C - Calculate the heat change during the experiment (3 mrks)
Heat change = McΔT
Mass, m = Density x volume
= 1g/cm3 x 75cm3
= 75g
= 0.075kh
ΔT = 27.5 − 20.0 = 7.5k
Hence, Heat change = 0.075 x 4.2 x 7.5
= 2.3625KJ - Work out the molar heat of neautralzation (1 mrk)
H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O (l)
Moles of ater formed = Moles of NaOH that reacted
= 2.0 x 50 = 0.1 moles
1000
0.1 mole of water → 2.325KJ of heat
∴ 1 mole of water → 1 x 2.3625 = 23.625 KJ
0.1
Hence ΔH = − 23.625 KJmole−1
2 = − 11. 8125KJmole−1 - Write the thermochemical ionic equation for this process (1 mrk)
2H+(aq) + 2OH−(aq) → 2H2O(l) ΔH = −23.625 KJ
*Accept H+(aq) + OH−(aq) → H2O(l) ΔH = −11.8125 KJmole−1 - Draw the energy level diagram for the process (1 mrk)
- State two sources of error in this experiment (2 mrk)
- Loss of heat to the environment
- Heat absorbed by the apparatus is not accounted for
- Errors in volume and temperature measurements
- Find T3 the common initial temperature (1 mrk)
- Define the term molar enthalpy of neutralization (1 mrk)
- Study the scheme given above and answer the questions that follow.
- Name the reagents used in :
Step I − Hydrogen gas (½ mrk)
Step II − Hydrogen chloride (½ mrk) - Name substances (2 mrks)
- Sodium propanoate
- Carbon (iv) oxide
- Water
- Bromoethane/1−bromoethane
- Write an equation for the reaction that takes place in: (3mrks)
Step IV − CH3CH2COONa(aq) + NaOH(aq) → C2H6(g) + Na2CO3(aq) (ignore state symbols) (1 mrk)
Step V − 2C2H2(g) + 5O2(g) → 4C02(g) + 2H2O(l)
Step VI − C2H6(g) + Br2 → C2H5Br(l) + HBr(g) - Explain one disadvantage of the continued use of items made from compound formed in step III
- It is non-biodegradable and hence a pollutant of the environment/produces poisonous gases when burnt.
- Name the type of reation that takes place in (2 mrks)
Step I − Addition/ Hydrogenation
Step III − Polymerization
Step V − Combustion/ Burning
Step Vi − Substitution - State the condition necessary for step II and III to take place. ( 2 mrks)
Step II − Presence of nickel catalyst
− Heat
Step III − High temeperature
− High pressure
- Name the reagents used in :
-
- The following diagrams shows the structure of two allotropes of carbon. Study them and answer the questions that follow:
- Name allotrope (1mrk)
M − Graphite
N − Diamond - Give on use of N (1mrk)
- In jewellery
- Making glass cutter and drilling bits.
- Which allotrope conducts electricity? Explain in terms of structure and bonding (2mrks)
- M/ Graphite − In graphite, every carbon atom is bonded to the other carbon atoms in a layer of hexagonal rings using three of the four valence electrons. The fourth valence electrons remains delocolized making graphite a good electrical conductor.
- Name allotrope (1mrk)
- In an experiment, carbon (iv) oxide gas was passed over heated charcoal and the gas produced collected as show in the diagram that follows;
- Write the equation for the reaction that place in the combustion tube. (1 mrk)
- CO2(g) + C (s) → 2CO(g)
- State the purpose of sodium hydroxide in the set up and explain how it works using a chemical equation. (2 mrk)
- To absorb the unreacted/ excess carbon(iv)oxide
- NaOH(aq) + C02(g) → NaHCO3(aq)
- Describe a simple chemical test that can be used to distinguish between carbon (iv) oxide and carbon(ii) oxide (2 mrk)
- Pass the gases separately through lime water. Carbon(iv) oxide forms a white precipitate but carbon(ii) oxide does not.
OR - Try to separately ignite them. Carbon (ii) oxide burns with a blue flame while CO2 does not.
- Pass the gases separately through lime water. Carbon(iv) oxide forms a white precipitate but carbon(ii) oxide does not.
- Give one use of carbon (ii) oxide (1 mrk)
- As a fuel
- As a reducing agent in extraction of metals
- Write the equation for the reaction that place in the combustion tube. (1 mrk)
- The following diagrams shows the structure of two allotropes of carbon. Study them and answer the questions that follow:
- The table below gives the standard reduction potentials of some elements represented by letters U, V, W, X and Z (They are not the actual symbols)
Element Standard electrode potentials (volts) -
- Identify the strongest reducing agent (1 mrk)
- U
- Which two half cells would produce the highest e.m.f (1 mrk)
- U and W
- Determine th e.m.f obtained from the cell above (2 mrk)
- Ecell = Ered − Eoxi
= + 0.79 − (− 2.36)
= + 3.15V
- Ecell = Ered − Eoxi
- What would element X represent? (1 mrk)
- Hydrogen or copper
- Identify the strongest reducing agent (1 mrk)
- Element V and Z were connected to form an electrochemical cell as shown in the diagram below
- Write the equation for the reaction that occurs at;
Metal Z elecrode (1 mrk)- Z(s) → Z2+(aq) + 2e
Metal V electrode (1 mrk) - V+(aq) + e → V(s)
- Z(s) → Z2+(aq) + 2e
- Write the cell representation for the above electrochemical cell (1 mrk)
- Z / Z2+ // 2V+/ V
- Determine the e.m.f of the above cell (1 mrk)
- Ecell = Ered − Eoxi
= + 0.34 −(− 0.76)
= +1.10V
- Ecell = Ered − Eoxi
- Write the overall cell reaction indicating the e.m.f ((1 mrk)
- Z2+(aq) + 2V(s) → Z2+(aq) + 2V
- State one use of a salt bridge and name two salts that can be used in the salt bridge
Use V − Used to complete the circuit between the two half cells (Any 1) (1 mrk)
− Balacing of charges
Salt − Potassium nitrate (1 mrk)
− Sodium nitrate
- Write the equation for the reaction that occurs at;
-
-
- The set-up below was used to react dry chlorine gas with iron powder. Thwe product Z was collected in flask B.
- Identify product Z
- Iron (ii) chloride/FeCl3
- What property of product Z makes it possible to be collected as shown in the diagram. (1 mrk)
- Sublimation property/Z sublimes
- Expalin why calcium oxide would be preferred to calcium(II) chloride to guard tube. (1 mrk)
- Calcium oxide can absorb both water vapour and excess/unreacted chlorine.
- The total mass of product Z formed was found to be 0.5g. Calculate the volume of chlorine gas that reacted with iron ( Fe = 56, Cl = 35.5, MG. V at 298K = 24,0000cm3) (3 mrks)
Fe(s) + 3Cl2 → 2FeCl3(s)
Moles of FeCl3 = 0.5
162.5
= 0.0030769 moles
Moles of chlorine = 0.0030769 x 3
2
= 0.00461535 moles
Volume of chlorine = 0.00461535 x 24000
= 110.7684cm3
- Identify product Z
- Name each processes described below which takes place when salt are exposed to air for sometime.
- Anhydrous copper (II) sulphate becomes wet (1 mrk)
- Hygroscopy
- Common table salt forms an aqueous solution (1 mrk)
- Deliquescence
- Fresh crystal of sodium carbonate, Na2C03.10H2O
- Effervescence
- Anhydrous copper (II) sulphate becomes wet (1 mrk)
- Study the scheme below and answer the question that follow
Identify solution Q and solid R- Solution Q (1 mrk)
- Aluminium sulphate
- Solid R (1 mrk)
- Barium sulphate
- Write an ionic equation for the reaction between solution Q and excess aqueous ammonia. (1 mrk)
- Al3+(aq) + 3OH−(aq) → Al(OH)3(s)
- Solution Q (1 mrk)
- The set-up below was used to react dry chlorine gas with iron powder. Thwe product Z was collected in flask B.
-
- The solubility in grammes of sodium nitrate in 100g of wterr are given below for various temperatures
Temp (°C) 10 20 30 40 50 60 70 80 90 100 Solubility(g/100g of water) - Plot a graph of solubility of sodium nitrate (y-axis) against temperature (x-axis) (3 mrk)
- Determine the temperature at which the solubility of the salt is 150g/100g of water (1 mrk)
- 85°C (½ mrk) Indicating on the graph - ½mrk
- Given 100g of a saturated solution of sodium nitrate at 100°C, calculate the mass of solute in the solution
180g − 100g
= 80g
- Plot a graph of solubility of sodium nitrate (y-axis) against temperature (x-axis) (3 mrk)
- Lead (II) sulphate can be prepared by double decomposition
- What is meant by double decomposition
- Reaction involving two soluble salts to give an insoluble salt and a soluble salt.
- Starting with 1.0M sodium sulphate descibe how you would prepare lead (II) sulphate solid. (2 mrk)
- Add lead (ii) nitrate solution to sodium sulphate solution to precipitate lead (ii) sulphate. Filter out the precipitate and dry between filter papers.
- What is meant by double decomposition
- A student analyzed a solution of salt L as follows.
Portion Test Observation I A few drops of dilute hydrochloric acid added to the solution No ppt II A few drops of dilute sulphuric (IV) acid White ppt II A few drops of lead(II) nitrate solution added and then warmed White precipitate which dissolves on warming - Identify the actions that were most probably present in the solution. (1 mrk)
- Ca2+ or Ba2+
- Write an ionic equation for the observation made in the test III (1 mrk)
- Pb2+(aq) + 2Cl−(aq) → PbCl2(s)
- Identify the actions that were most probably present in the solution. (1 mrk)
- The solubility in grammes of sodium nitrate in 100g of wterr are given below for various temperatures
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Paper 2 Questions and Answers - BSJE Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students