Instructions:
- Answer ALL questions
- You are NOT allowed to start working with the apparatus for the first 15 minutes of the 2 ¼ hours allowed for this paper. This time will enable you read through the question paper and make sure you have all the chemicals and apparatus required.
- Mathematical tables and electronic calculators may be used
- All working must be clearly shown where necessary.
1.You are provided with:
- Solution A of Potassium manganate (VII).
- 0.05M solution B of oxalic acid.
- Solution C containing 4.9g of ammonium iron (II) Sulphate, (NH4)2 SO4.FeSO4.6H2O, in 250cm3 of water.
- You are required to:
- Determine the rate of reaction between oxalic acid and Potassium manganate (VII) solutions.
- Standardize the solution A.
PROCEDURE I:
- Fill the burette with solution A.
- Place 1 cm3 of solution A from the burette into each of the five (5) test-tubes in a test tube rack.
- Using a clean measuring cylinder, place 19 cm3 of solution B into a boiling tube.
- Place the thermometer into solution B and heat gently until it attains a temperature of 40°C.
- Add the first portion of solution A immediately and at the same time start a stop watch.
- Record the time taken for solution A to be decolorized in table I below.
- Repeat the procedure (i) to (v) at temperatures of 50°C,60°C,70°C and 80°C to complete the table.
Table I
| Temperature of solution B (oC) | 40 | 50 | 60 | 70 | 80 |
| Time taken for decolorization (seconds) | |||||
| Rate ( I/t ) s-1 X 1000 |
(4 marks)
- Plot a graph of ( 1/t X 1000) against temperature (X-axis) (3 marks)
- From the graph determine the time taken for the mixture to decolourise at 65°C (2 marks)
- How does the rate of reaction between oxalic acid solution B and Potassium manganate (VII) solution A vary with temperature? (1 mark)
PROCEDURE II
- Refill the burette with solution A.
- Pipette 25cm3 of solution C into a conical flask and titrate the solution A against solution C until a permanent pink colour just appears.
- Record your results in table II below and repeat the procedure to fill the table.
Table II
1 2 3 Final burette reading (cm3) Initial burette reading (cm3) Volume of A used (cm3)
- Determine the average volume of A used. (1 mark)
- Calculate the concentration of solution C in moles per litre (Fe = 56, S = 32, O = 16, N =1 4, H = 1) (1 mark)
- Find the number of moles of solution C used. (1 mark)
- Given the ionic equation for the reaction is
5Fe2+(aq) + MnO4−(aq) + 8H+(aq) ⟶ 5Fe3+(aq) + Mn2+(aq) + 4H2O(l);
Find the number of moles of solution A used. (1 mark) - Determine the concentration of the Potassium manganate (VII), solution A in moles per litre. (2 marks)
2.You are provided with solid Q. Carry out the tests below and record your observations and inferences in the table below
- Place half a Spatula full of solid Q in a clean dry test-tube and heat gently then Strongly.
Test the gas produced using moist red and blue litmus papers
Observations Inferences (1 mark) (1 mark) - Place the remaining solid Q in a boiling tube, add about 5cm3 of distilled water and shake well. Divide the resulting mixture into four portions for the tests below.
Observations Inferences (1 mark) (1 mark) - To the first portion add Sodium hydroxide solution dropwise until in excess.
Observations Inferences (1 mark) (1 mark) - To the second portion add 2-3 drops of dilute Sulphuric (VI) acid
Observations Inferences (1 mark) (1 mark) - To the third portion add aqueous ammonia dropwise until in excess
Observations Inferences (1 mark) (1 mark) - To the fourth portion add 2-3 drops acidified barium nitrate solution
Observations Inferences (1 mark) (1 mark)
- To the first portion add Sodium hydroxide solution dropwise until in excess.
3.You are provided with solid L. carry out the tests below on L and record the observations and inferences in the spaces provided.
- Place half of solid L in a boiling tube and add about 5cm3 of distilled water
Divide the solution into two portions and carry out the tests below.Observations Inferences (1 mark) (1 mark) - To the first portion add 2-3 drops of acidified potassium manganate (VII).
Observations Inferences (1 mark) (1 mark) - To the second portion add Sodium carbonate provided.
Observations Inferences (1 mark) (1 mark)
- Place the remaining solid L in metallic spatula and ignite it.
Observations Inferences (1 mark) (1 mark)
CONFIDENTIAL
- Apart from the school laboratory fittings supply the following
- 100cm3 solution A - 0.02M Acidified potassium manganite (VII) (H+/KMnO4)
- 100cm3 Solution B - 0.05M Oxalic acid
- 100cm3 Solution C - containing 4.9g of (NH4)2SO4 FeSO4.6H2O in 250cm3 of solution)
- 50ml measuring cylinder
- 5 Test tubes
- 1 boiling tube
- 250ml beaker
- Thermometer
- Stopwatch
- Burette
- Pipette
- Two conical flasks
- About 0.5g of solid Q (ZnSO4)
- Distilled water
- About 0.5g of solid L (oxalic acid)
- Red and blue litmus papers
- Metallic spatula
- test tube holder
- 0.5g sodium carbonate
ACCESS TO:
- 1 M Sodium hydroxide solution.
- 1M Ammonia solution.
- 0.5M Acidified Barium Nitrate solution.
- 1M sulphuric (VI) acid.
- Acidified potassium Manganate (VII)
- Source of heat.
NB
- Solution A 0.02M of H+/KMnO4 is prepared by dissolving 3.16g of KMnO4 in 400cm3 of 2M H2SO4. Add distilled water to make 1 litre of solution.
- 1M sodium hydroxide is prepared by dissolving 40 g of sodium hydroxide dissolved in about 200cm3 of distilled water in a 1000 ml volumetric flask and top up with distilled water to the mark.
- Acidified Barium Nitrate solution is prepared by dissolving 26.1g of Barium Nitrate in 400cm3 of 2M nitric acid and top up to 1 litre of solution using distilled water.
- 2M Sulphuric acid. Prepared by dissolving 110cm3 of concentrated Sulphuric acid in about 600cm3 of distilled water and diluting to one litre of solution.
- Solution B 0.05M oxalic prepared by dissolving 6.3g of dehydrated oxalic acid in about 400ml of distilled water in a 1 litre volumetric flask and top up to the mark.
MARKING SCHEME
Q1. Table I/procedure I
Complete table – (1mark)
- 5 values -1mark
- 3-4 values ½ mark
- 1-2 values 0 mark
Decimal place.
- Tied to the time
Accept time to 2 decimals, 1 decimal or whole number used consistently
Accuracy (1mk)
- Compare the first value with teacher’s value.
If within range of ±2 units award 1 mark otherwise award 0 mark if outside the range
Trend – (1mark)
- Continuous decrease in time (1mark)
penalize fully for any inconsistence in the trend
Graph (3mark)
- Scale – ½ mark
Occupy at least 8 squares on both axes
Labelling axes – ½ mark
- Both axes labelled correctly showing correct variables- ½ mark
(Units may or may not be given. But if given must be correct. Otherwise penalize fully for wrong units
Plotting 1 mark
- 4-5 correctly plotted points -1mark
- 2-3 correctly plotted points – ½ mark
Shape/curve-1mark
Smooth curve – 1mark
(ii) Correct showing on graph√1 1mark. Correct reading from graph – (1 mark)
(iii). Rate of reaction increase with increase in temperature√ 1mark
Procedure II
Table
- Complete Table (C.T- 1 mark
- C.T with 3 titrations 1 mark
- C.T with 2 titrations 1 mark
- C.T with 1 0 mark
Penalties
- Inverted table penalize ½ mark
- Wrong arithmetic ½ mark
- Unrealistic values below 1cm3 and above 50cm3 penalize ½ mark
- NB/ penalize to a maximum of ½ mark
Decimal place (D.P) (1mark)
- Values to 1 d.p or 2 d.p of 25, 50, 75 i.e
- 2nd d.p should be 0 or 5
Accuracy (A) – 1mark
- If any value is within the range 0.1 of school value award 1 mark
- If any value is within the range of 0.2 of school value award ½ mark
Principle of averaging (P.A) – 1mark
- Average value that are 0.2 of each other (1mark)
Final answer/accuracy (F.A) – 1mark
- Compare the averaged value with school value.
- If within range of 01 1mk, if range of 0.2 ½ mark
(ii). RFM of (NH4)2SO4. FeSO4. 6H2O = 392
∴concentration = 19.6/392√1
= 0.05moles√1 (2marks)
(iii). Moles of C used = (1mark)
0.05 x 25 √½ = 0.00125 moles√½
1000
(iv). Moles of A used = 1/5 x 0.00125½(1mark)
= 0.00025 moles √½
(v) Average tire → 0.00025 moles (1mark)
1000cm3 →
0.00025 x 1000√1 = correct answer √1
Average tutre
Q2.
(i).
| Observations | Inferences |
|
|
(ii)
| Observations | Inferences |
|
|
(a).
| Observations | Inferences |
|
|
(b).
| Observations | Inferences |
|
|
(c)
| Observations | Inferences |
|
|
(d).
| Observations | Inferences |
|
|
Q3.
(a)
(i)
| Observations | Inferences |
|
|
(ii).
| Observations | Inferences |
|
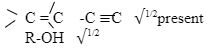 |
(iii).
| Observations | Inferences |
|
|
(b).
| Observations | Inferences |
|
Download Chemistry Paper 3 Questions and Answers with Confidential - Lainaku II Joint Mock Examination 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
