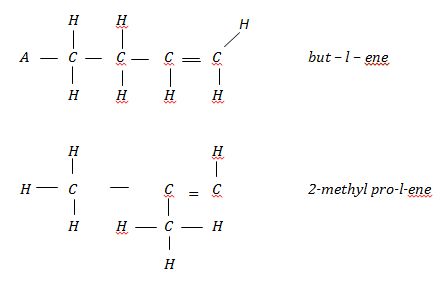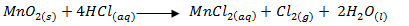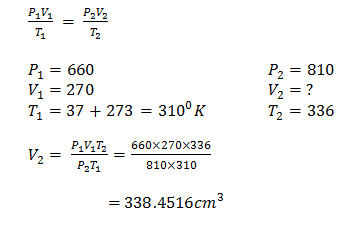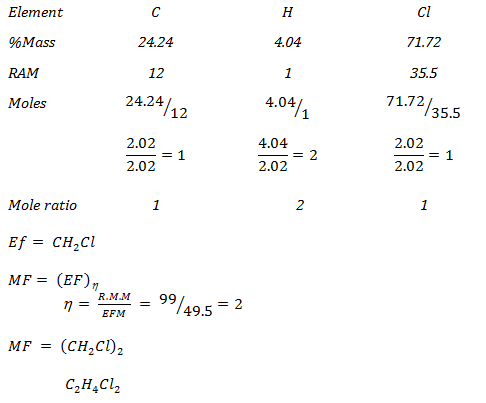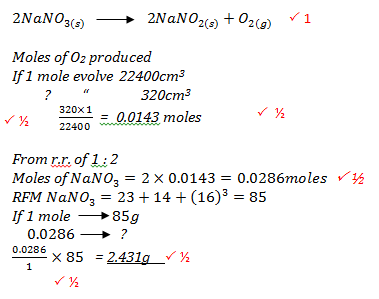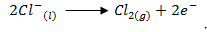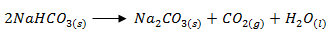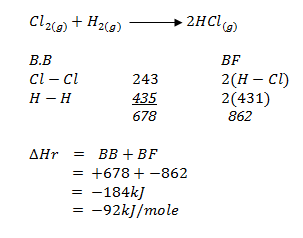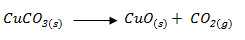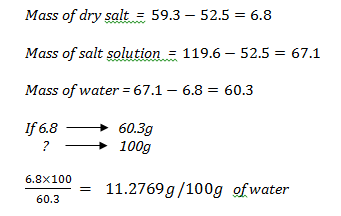- State two laboratory rules that should be followed to avoid contamination and wastage of chemicals. (2 marks)
-
- Give one reason some of the laboratory apparatus are made of ceramics. (1 mark)
- Name two apparatus that can be used to measure approximately 75 cm3 of dilute sulphuric (VI) acid. (2 marks)
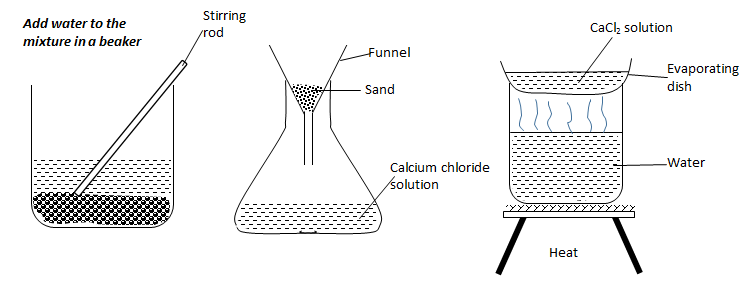
- Draw the procedural set-ups that can be used to separate a mixture of sand and calcium chloride to obtain crystals of calcium chloride. (3 marks)
- State two applications of chromatography. (2 marks)
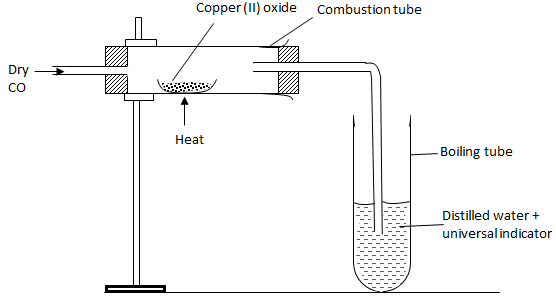
- The below set-up was used to determine the chemical properties of carbon (II) oxide.
- Write the chemical equation for the reaction taking place in the combustion tube.(1 mark)
- State and explain the observation made in the boiling tube. (2 marks)
- A student placed some hydrogen peroxide in a test tube then added a small amount of manganese (IV) oxide. A glowing splint was then brought near the mouth of the tube.
- State the observation made on the glowing splint. (1 mark)
- What is the role of the manganese (IV) oxide? (1 mark)
- Give one use of the gas produced. (1 mark)
- An organic compound with formular has isomers. Draw and name two possible structural isomers of the compound. (3 marks)
- Explain how the compound and can be distinguished using bromine water. (2 marks)
-
- Chlorine can be prepared in the laboratory by using the following reagents and chemicals.
Concentrated sulphuric (VI) acid, water, manganese (IV) oxide, concentrated hydrochloric acid.- State the role of concentrated sulphuric (VI) acid. (1 mark)
- Write the equation for formation of chlorine. (1 mark)
- What is the role of manganese (IV) oxide? (1 mark)
- Chlorine can be prepared in the laboratory by using the following reagents and chemicals.
-
- State Boyle’s law. (1 mark)
- A gas occupies 270cm3 at a pressure of 660mmHg at 370C. What is the new volume if pressure is changed to 810 mmHg at 630 C? (2 marks)
- An organic compound contains 24.24% carbon, 4.04% hydrogen and the rest chlorine. If its relative molecular mass is 99, what is its molecular formula? (3 marks)
(C = 12, H = 1, Cl = 35.5)
- A given mass of sodium nitrate was heated completely and 320 cm3 of the gas was produced at s.t.p. Determine the mass of the sodium nitrate heated.
(Na = 23. N = 14, O = 16, molar gas volume = 22.4L) (3 marks)
-
- Give one advantage of using methyl orange over phenolphthalein as an indicator.(1 mark)
- Three drops of litmus solution was added to 20 cm3 of 2M hydrochloric acid in a beaker followed by 20 cm3 of 2M ammonium hydroxide. State and explain the observation made. (2 marks)
- A tea farmer suspects that her farm had turned acidic. She obtained a soil sample to analyze for pH. Give her the procedure to follow in order to verify this. (2 marks)
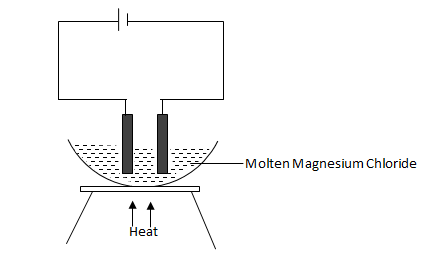
- Study the diagram below and answer the questions that follow.
- Define electrolysis. (1 mark)
- On the diagram, label the Anode and Cathode. (2 marks)
- Write the equation at the anode. (1 mark)
- In order to find the proportion by volume of gases in air, a sample of air was passed through two wash bottles, the first containing sodium hydroxide solution and the second containing concentrated sulphuric (VI) acid. The remaining gas was then collected in a syringe.
- Why was the air passed through;
- sodium hydroxide solution? (1 mark)
- concentrated sulphuric (VI) acid? (1 mark
- Name the major gas collected in the syringe. (1 mark)
- Why was the air passed through;
- During the manufacture of sodium carbonate in the industry.
- Give the name of the process to manufacture sodium carbonate. (1 mark)
- Write the final equation for the formation of sodium carbonate during the process. (1 mark)
- Give one use of sodium carbonate. (1 mark)
- Describe how to prepare crystal of magnesium sulphate starting with magnesium powder. (3 marks)
-
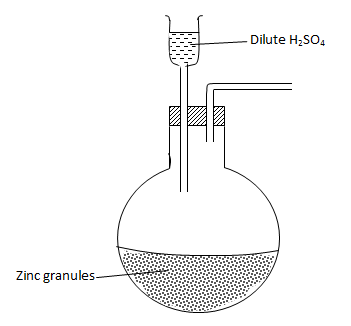
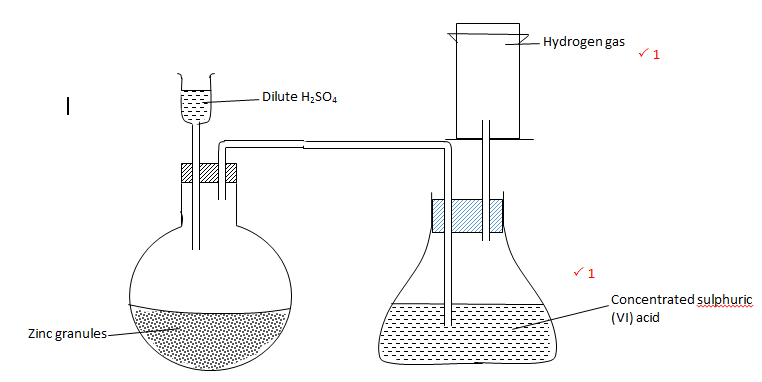
- Complete the diagram below to show how dry sample of hydrogen gas is prepared in the laboratory. (2 marks)
- Name the catalyst which could be used to increase the reaction rate of production of hydrogen gas in the set up drawn above. (1 mark)
- Complete the diagram below to show how dry sample of hydrogen gas is prepared in the laboratory. (2 marks)
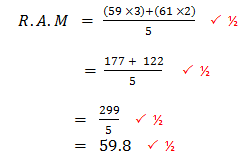
- An element consists of two isotopes with atomic masses 59 and 61 in the ratio of 3 : 2 respectively.
- What are isotopes? (1 mark)
- Calculate the relative atomic mass of the element. (2 marks)
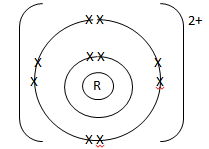
- An element
:
- To which chemical family does it belong? (1 mark)
- Write the electron arrangement of the atom. (1 mark)
- Draw the structure of its ion. (1 mark)
- Given the bond energies.
H – Cl 431kJ/mole
H – H 435kJ/mole
Cl – Cl 243kJ/mole- Calculate the enthalpy change for the formation of hydrogen chloride gas when chlorine and hydrogen react. (2 marks)
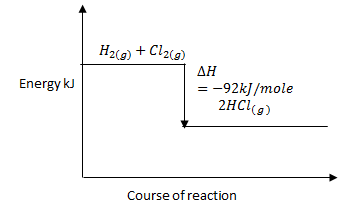
- Sketch the energy level diagram for the reaction. (1 mark)
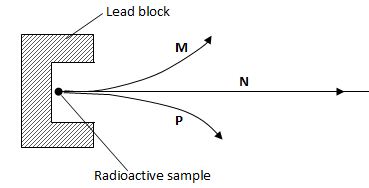
- The diagram below shows the radiations emitted by a radioactive sample.
Name the radiations; P, M, N (3 marks)
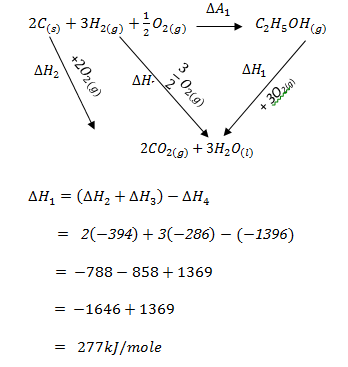
- Calculate the enthalpy of formation of ethanol given the enthalpies of;
combustion of ethanol = -1369 kJ/mole
combustion of carbon = -394kJ/mole
combustion of hydrogen = -286kJ/mole (3 marks)
-
- State what is observed when sodium hydroxide pellets are left in air overnight. (1 mark)
- What name is given the process shown by the salt in (a) above? (1 mark)
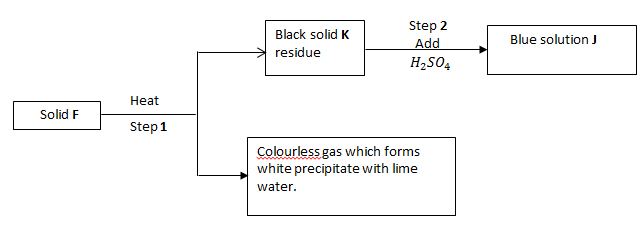
- Given;
- Identify;
Solid F (1 mark)
Solid J (1 mark) - Write equation for step 1. (1 mark)
- Identify;
- A saturated solution of sodium nitrate in water was made at 300C. Use the information below to answer the questions that follow.
Mass of evaporating dish = 52.5g
Mass of evaporating dish + salt solution = 119.6g
Mass of evaporating dish + dry salt = 59.3g- What is solubility? (1 mark)
- Determine the solubility of sodium nitrate at 300C. (2 marks)
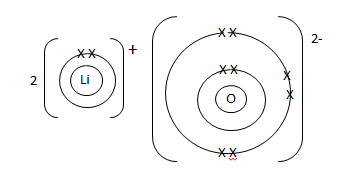
- Use dot (·) and cross (X) to show the bonding in Lithium oxide. (1 mark)
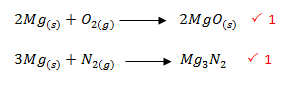
- Excess magnesium ribbon was burnt in air to form a white solid mixture. Write two equations to show the formation of the white solid mixture. (2 marks)

MARKING SCHEME
- State two laboratory rules that should be followed to avoid contamination and wastage of chemicals. (2 marks)
- Label all containers carrying chemicals.
- Turn off water and gas taps when not in use.
- Always work on a clean bench.
- Label the chemicals you are using before an experiment.
-
- Give one reason some of the laboratory apparatus are made of ceramics. (1 mark)
Does not break easily hence can withstand strong heating.
- Name two apparatus that can be used to measure approximately 75 cm3 of dilute sulphuric (VI) acid. (2 marks)
- 100cm3 measuring cylinder.
- Graduated 100cm3
- Give one reason some of the laboratory apparatus are made of ceramics. (1 mark)
- Draw the procedural set-ups that can be used to separate a mixture of sand and calcium chloride to obtain crystals of calcium chloride. (3 marks)
- State two applications of chromatography. (2 marks)
- In sports to identify banned substances.
- To test purity of drugs in pharmacy.
- Identify contaminants in food and drinks.
- Identify harmful substances in cosmetics.
- The below set-up was used to determine the chemical properties of carbon (II) oxide.
- Write the chemical equation for the reaction taking place in the combustion tube.(1 mark)
CO(g)+CuO(s)→Cu(s)+CO2(g) - State and explain the observation made in the boiling tube. (2 marks)
The solution turns red, CO2 formed dissolves to form an acidic solution.
- Write the chemical equation for the reaction taking place in the combustion tube.(1 mark)
- A student placed some hydrogen peroxide in a test tube then added a small amount of manganese (IV) oxide. A glowing splint was then brought near the mouth of the tube.
- State the observation made on the glowing splint. (1 mark)
It relit.
- What is the role of the manganese (IV) oxide? (1 mark)
Serves as a catalyst.
- Give one use of the gas produced. (1 mark)
- Breathing aid by patients with respiratory ailment.
- Oxyhydrogen flame is used in welding and cutting metals.
- Mixed with helium for deep sea diving and mountain climbing
- State the observation made on the glowing splint. (1 mark)
- An organic compound with formular has isomers. Draw and name two possible structural isomers of the compound. (3 marks)
- Explain how the compound and can be distinguished using bromine water. (2 marks)
Bubble the 2 compounds separating in bromine water will decolourize the yellow bromine in water while C4H10 will not decolorize the bromine water.
-
- Chlorine can be prepared in the laboratory by using the following reagents and chemicals.
Concentrated sulphuric (VI) acid, water, manganese (IV) oxide, concentrated hydrochloric acid.- State the role of concentrated sulphuric (VI) acid. (1 mark)
To dry chlorine
- Write the equation for formation of chlorine. (1 mark)
- What is the role of manganese (IV) oxide? (1 mark)
Oxidizes HCl to chlorine
- State the role of concentrated sulphuric (VI) acid. (1 mark)
- Chlorine can be prepared in the laboratory by using the following reagents and chemicals.
-
- State Boyle’s law. (1 mark)
For a fixed mass of a gas, the volume is inversely proportional to the pressure at constant temperature.
- A gas occupies 270cm3 at a pressure of 660mmHg at 370C. What is the new volume if pressure is changed to 810 mmHg at 630 C? (2 marks)
- State Boyle’s law. (1 mark)
- An organic compound contains 24.24% carbon, 4.04% hydrogen and the rest chlorine. If its relative molecular mass is 99, what is its molecular formula? (3 marks)
(C = 12, H = 1, Cl = 35.5)
- A given mass of sodium nitrate was heated completely and 320 cm3 of the gas was produced at s.t.p. Determine the mass of the sodium nitrate heated.
(Na = 23. N = 14, O = 16, molar gas volume = 22.4L) (3 marks)
-
- Give one advantage of using methyl orange over phenolphthalein as an indicator.(1 mark)
Shows distinct colours is acids, bases and neutral solutions unlike phenolphthalein which cannot differentiate between acids and neutral solutions.
- Three drops of litmus solution was added to 20 cm3 of 2M hydrochloric acid in a beaker followed by 20 cm3 of 2M ammonium hydroxide. State and explain the observation made. (2 marks)
The colour changed from red to colourless.
The acid was neutralized completely by the ammonium hydroxide.
- Give one advantage of using methyl orange over phenolphthalein as an indicator.(1 mark)
- A tea farmer suspects that her farm had turned acidic. She obtained a soil sample to analyze for pH. Give her the procedure to follow in order to verify this. (2 marks)
- Mix the sample of soil with distilled water and shake.
- Decant or filter to obtain a solution.
- Add a few drops of universal indicator to a test tube containing the soil sample solution.
- Observe the colour and compare with pH chart to read out the pH.
- Study the diagram below and answer the questions that follow.
- Define electrolysis. (1 mark)
Process by which an electrolyte gets decomposed when an electric current is passed through it.
- On the diagram, label the Anode and Cathode. (2 marks)
- Write the equation at the anode. (1 mark)
- Define electrolysis. (1 mark)
- In order to find the proportion by volume of gases in air, a sample of air was passed through two wash bottles, the first containing sodium hydroxide solution and the second containing concentrated sulphuric (VI) acid. The remaining gas was then collected in a syringe.
- Why was the air passed through;
- sodium hydroxide solution? (1 mark)
To absorb CO2
- concentrated sulphuric (VI) acid? (1 mark
To absorb water vapour
- sodium hydroxide solution? (1 mark)
- Name the major gas collected in the syringe. (1 mark)
Nitrogen
- Why was the air passed through;
- During the manufacture of sodium carbonate in the industry.
- Give the name of the process to manufacture sodium carbonate. (1 mark)
Solvay process
- Write the final equation for the formation of sodium carbonate during the process. (1 mark)
- Give one use of sodium carbonate. (1 mark)
- Manufacture glass
- softening hard water
- Give the name of the process to manufacture sodium carbonate. (1 mark)
- Describe how to prepare crystal of magnesium sulphate starting with magnesium powder. (3 marks)
- To some amount of dilute sulphuric (VI) acid in beaker
- Add magnesium powder as you stir till in excess.
- Filter to obtain magnesium sulphate as filtrate.
- Heat the filtrate to concentrate.
- Cool in order to form crystals.
- Dry between filter papers.
-
- Complete the diagram below to show how dry sample of hydrogen gas is prepared in the laboratory. (2 marks)
- Name the catalyst which could be used to increase the reaction rate of production of hydrogen gas in the set up drawn above. (1 mark)
Crystals of copper (II) sulphate
- Complete the diagram below to show how dry sample of hydrogen gas is prepared in the laboratory. (2 marks)
- An element consists of two isotopes with atomic masses 59 and 61 in the ratio of 3 : 2 respectively.
- What are isotopes? (1 mark)
Atoms of the same element with same atomic number but different mass number due to difference in the number of neutrons.
- Calculate the relative atomic mass of the element. (2 marks)
- What are isotopes? (1 mark)
- An element
:
- To which chemical family does it belong? (1 mark)
Alkaline earth metals
- Write the electron arrangement of the atom. (1 mark)
2.8.2
- Draw the structure of its ion. (1 mark)
- To which chemical family does it belong? (1 mark)
- Given the bond energies.
H – Cl 431kJ/mole
H – H 435kJ/mole
Cl – Cl 243kJ/mole- Calculate the enthalpy change for the formation of hydrogen chloride gas when chlorine and hydrogen react. (2 marks)
- Sketch the energy level diagram for the reaction. (1 mark)
- Calculate the enthalpy change for the formation of hydrogen chloride gas when chlorine and hydrogen react. (2 marks)
- The diagram below shows the radiations emitted by a radioactive sample.
Name the radiations; P, M, N (3 marks)
P - Alpha
M - Beta
N - Gamma rays
- Calculate the enthalpy of formation of ethanol given the enthalpies of;
combustion of ethanol = -1369 kJ/mole
combustion of carbon = -394kJ/mole
combustion of hydrogen = -286kJ/mole (3 marks)
-
- State what is observed when sodium hydroxide pellets are left in air overnight. (1 mark)
It forms a solution.
- What name is given the process shown by the salt in (a) above? (1 mark)
Deliquescence
- State what is observed when sodium hydroxide pellets are left in air overnight. (1 mark)
- Given;
- Identify;
Solid F - Cu CO3 (1 mark)
Solid J - CuSO4 (1 mark) - Write equation for step 1. (1 mark)
- Identify;
- A saturated solution of sodium nitrate in water was made at 300C. Use the information below to answer the questions that follow.
Mass of evaporating dish = 52.5g
Mass of evaporating dish + salt solution = 119.6g
Mass of evaporating dish + dry salt = 59.3g- What is solubility? (1 mark)
Amount of solute that can form a saturated solution in 100g of water at a given temperature.
- Determine the solubility of sodium nitrate at 300C. (2 marks)
- What is solubility? (1 mark)
- Use dot (·) and cross (X) to show the bonding in Lithium oxide. (1 mark)
- Excess magnesium ribbon was burnt in air to form a white solid mixture. Write two equations to show the formation of the white solid mixture. (2 marks)
Download CHEMISTRY PAPER 1 - KCSE 2019 MOKASA PRE MOCK EXAMINATION.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students