Instructions to students
- Write your name, Admission number, school and class in the space provided above.
- All working must be shown clearly in the space provided.
- Non programmable silent electronic calculators may be used.
- Students should check the questions paper to ascertain that all the pages are printed.
- Students should answer the questions in English.
- Answer ALL questions in the spaces provided.
FOR EXAMINERS USE ONLY
|
QUESTION |
MAXIMUM SCORE |
CANDIDATES’ SCORE |
|
1-28 |
80 |

QUESTIONS
- Using reagents provided only, explain how you could prepare a salt of Zinc carbonate solid. Dilute nitric(v) acid, zinc, sodium carbonate (3mks)
- The diagram below shows a Bunsen burner when in use
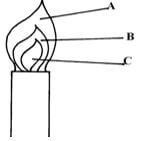
Describe an experiment that would confirm that region labeled C is unsuitable for heating. (2mks). -
- On the grid provided sketch a graph of pressure against volume for fixed mass of a gas at constant temperature (1mk)

- A fixed mass of a gas has a volume of 250cm3 at 27oC and 750mmHg pressure.
Calculate the gas volume that the gas would occupy at 41oC and 750mmHg pressure. (0o = 273k) (2mks)
- On the grid provided sketch a graph of pressure against volume for fixed mass of a gas at constant temperature (1mk)
- 22.2cm3 of sodium hydroxide solution containing 4.0g per litre sodium hydroxide were required for complete neutralisation of 0.1g of a dibasic acid. Calculate the relative formula mass of the dibasic acid. (Na = 23, O=16, H=1) (3mks)
- The diagram below represents a laboratory experiment to investigate the reaction between hydrogen - sulphide gas and an aqueous iron (III) chloride.
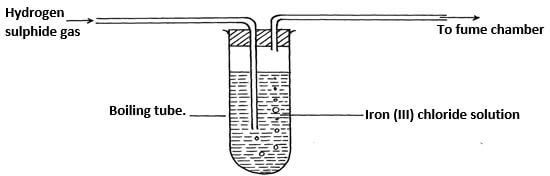
- Write chemical equation for the reaction which takes place in the boiling tube. (1mk)
- What adjustment need to be made in the above set-up if the laboratory does not have a fume chamber. (1mk)
- Describe a laboratory chemical test for a sample of hydrogen sulphide gas. (1mk)
- A group of compounds called chlorofluorocarbons have a wide range of uses but they have harmful effects on the environment. State and explain one harmful effect of chlorofluorocarbons on the environment. (2mks)
- X grams of a radioactive isotope takes 120 days to decay to 3.5 grams. The half-life period of the isotope is 20 days.
- Calculate the initial mass of the isotope (2mks)
- State the application of radioactivity in agriculture. (1mk)
- Sulphur and sodium belong to the same period on the periodic table. State and explain the difference in M.P of the oxide of sulphur and the oxide of sodium. (3mks)
-
- Water is an example of a polar solvent. What is a polar solvent? (1mk)
- Explain the following observations HCl gas dissolves in water to form an electrolyte, while the same chloride dissolves in methylbenzene to form a non-electrolyte (1mk)
-
- Define the term deposition (1mk)
- Describe how you can obtain copper powder from a mixture containing copper and zinc powder. (2mks)
-
- Name the main ore from which iron is extracted. (1mk)
- Name two substances that convert iron (III) oxide to iron in the blast furnace. (2mks)
-
- Write an equation showing how boiling can remove temporary water hardness.(1mk)
- Name one method that can be used to remove both temporally and permanent water hardness. (1mk)
- Other than wastage of soap during cleaning, state one other disadvantage of hard water.(1mk)
-
- Name two pure allotropes of carbon. (1mk)
- State and explain using relevant equations the observation made when carbon(IV) oxide is bubbled through calcium hydroxide solution for a long time. (2mks)
- When Na2CO3.xH2O is strongly heated it loses 63.2% of its mass. Determine the value of x in the compound(Na = 23, O = 16, H = 1) (3mks)
- Dry ammonia was passed over a heated lead(II) oxide in a combustion tube as shown
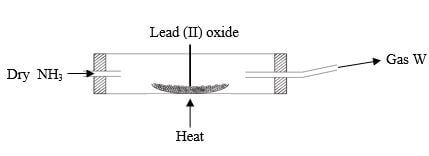
- What observations would be made in the combustion tube (1mk)
- Write a chemical equation for the reaction in the combustion tube (1mk)
- State one industrial use of ammonia (1mk)
- An ion of P2+ has a configuration of 2.8
- Name the family to which P belong (1mk)
- Compare the atomic and ionic radius of P. Explain (2mks)
-
- Explain why alkanes are used as fuel (1mk)
- Draw the structure of the following compounds (2mks)
- 3-methylbut – 1 yne
- But – 2 –ene
-
- Define solubility (1mk)
- Study the information in the table and answer the questions below
Salt
Solubility (g) 100g water
At 400C
At 600C
CUSO4
28
38
Pb(NO3)2
79
98
- Calculate the mass of CuSO4 that would saturate 200g of water at 60ºC (1mk)
- A solution containing 80g of Pb(NO3)2 in 100g of water at 600C was cooled to 40ºC. Calculate the mass of Pb(NO3)2 that crystallized (1mk)
- Dilute hydrochloric acid was added to a compound Z of copper. The solid reacted with the acid to form a colourless gas which formed a white precipitate when bubbled through lime water.
- Name solid Z (1mk)
- State the observation that would be made if a similar compound of lead is used. Explain. (2mks)
-
- Explain why the reactivity of group(VII) elements decrease down the group (2mks)
- Moist blue litmus and dry blue litmus paper were introduced into gas jars of dry chlorine. State the observations that would be made. (1mk)
-
- Name the reagents that are commonly used in the preparation of hydrogen (1mk)
- Study the diagram below and answer the questions that follow
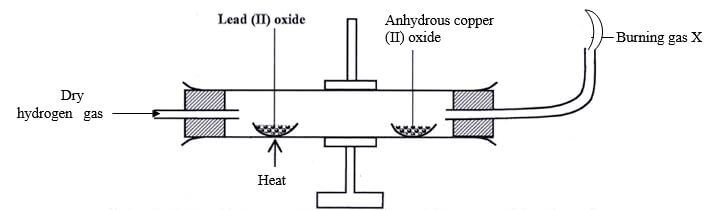
- Name gas x (1mk)
- State and explain the observation made in the anhydrous copper(II) sulphate after sometime (1mk)
-
- State two physical properties of sulphur (IV) oxide (1mk)
- Explain why when sulphur (IV) oxide is bubbled into acidified potassium dichromate (VI) the solution changes colour from orange to green. Explain the observation (1mk)
- In the contact process of manufacture of sulphuric(VI) acid, explain how pollution by SO2 is reduced. (1mk)
- Study the setup below and answer questions that follow
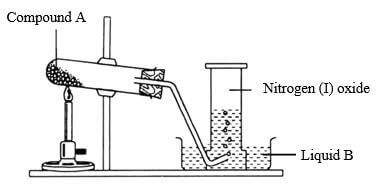
- Name (1mk)
- Compound A
- Liquid B
- Why is the boiling tube tilted downwards (1mk)
- Name (1mk)
- Explain why
- Aluminium is commonly used for making cooking pots and pans. (1mk)
- Silicon(IV) oxide is a poor conductor of heat and electricity (1mk)
- The set up below was used to show electrolysis in molten lead(II) iodide
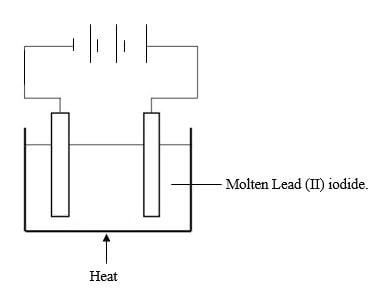
- On the diagram label the cathode (1mk)
- State the observation that was made at the anode during the electrolysis. Give a reason for your answer (2mks)
- 100cm3 of carbon (II) oxide gas was reacted with 100cm3 of oxygen. (All volume were measured under the same conditions of temple and pressure.
- Determine
- Volume of the product formed (1mk)
- The gas which was in excess and by what volume (2mks)
- Determine
-
- Using a dot(.) and cross(x) diagram of carbon(II) oxide, differentiate between a covalent and a co-ordinate bond (1mk)
- Use dot (.) and cross(x) diagrams to show bonding in between the elements represented by the following symbols. (2mks)
- 2412X and 199Y
- Study the flow diagram below
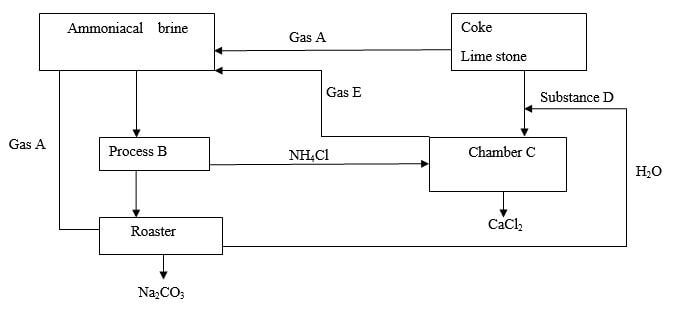
- Name
- Gas A (½ mk)
- Process B (½ mk)
- Substance D (½ mk)
- Gas E (½ mk)
- Write the equation for the reaction in chamber C (1mk)
- Name
MARKING SCHEME
- Into a given amount of dilute nitric (V) acid in a beaker add excess Zinc powder and stir√ (½mk) (until no more can dissolve). Filter the mixture to obtain unreacted zinc powder as the residue and zinc nitrate solution as the filtrate. √ (½mk) Into a beaker containing water add solid sodium carbonate and using a stirring rod stir until all of it dissolves. √ (½mk) Into the beaker containing zinc nitrate solution add the sodium carbonate solution. Zinc carbonate will be precipitated out. √ (½mk) Filter the mixture to obtain zinc carbonate as the residue. √ (½mk) Rinse the residue using distilled water to remove impurities the dry it in between two dry filter papers. √ (½mk)
- Hold a match stick on a pin and let the head rest on the chimney when the chimney is lit the head of the match stick in the zone C does not light.
Or
Place a wooden splint or a card board across the region momentally it is noted that the region was not charred while the outer region was charred - P1V1 = P2V2; P1 = 750 V1 = 250, T1 = 27 + 273 = 300k
T1 T2
P2 = 750, T2 = 41 + 273 = 311k
V2 = P1V1T2 = 750 x 250 x 311 √ (1mk) = 311x 5
T1 x P2 300 x 750 6
= 259.167cm3√ (1mk) - Molarity of NaOH = 4/40 = 0.1M √ (½mk)
Moles in 22.2 cm3 = 22.2 X 0.1
1000
= 0.00222 √ (½mk)
Mole ratio OH- : H+ √ (½mk)
2 : 1
moles of acid = 0.00222
2
= 0.00111 √ (½mk)
mass = 1 x 0.1 √ (½mk)
0.00111
= 90 √ (½mk) -
- H2S(g) + 2FeCl3(aq) → 2FeCl2(aq) + S(s) + 2H+(aq)
-
- Pass excess gas, H2S through ecess concentrated sulphuric acid √ (½mk) to absorb H2S gas. Through an alkali or 3H2S(s) + H2SO4(l) → 4S(s) + 4H2O(l)
- Hydrogen sulphide gas is poisonous √ (½mk)
-
- Pass H2S through Lead (II) nitrate solution √ (½mk) a black precipitate of lead (II) sulphide is formed √ (½mk)
- They destroy the ozone layer in the atmosphere. √ (1mk) Ozone layer absorb harmful ultra violet light rays from the sun√ (½mk) and once destroyed the harmful sun rays reach the earth which may cause cancer, eye problems and √ (½mk)
-
- 120/20 = 6(half litres)
3.5 x 26
y = 224grams - Study the rate of absorption of fertilizer by plants using radioactive phosphorous
tracing chemical and physiological processes such as photosynthesis by use of radioactive carbon 14
- 120/20 = 6(half litres)
- Oxide of sodium has higher M.P than the oxide of sulphur because oxide of sodium has a giant ionic structure where a lot of energy is required breakionic bond while oxide of sulphur has simple molecular structure where little energy is required to break van der waals forces.
-
- A solvent whose molecules behaves as if its negatively charged on one end and positively charged in the opposite end. Solvent whose molecules are partially charged
- When dissolved in water dissociate √ ½ into hydrogen and chloride ions hence an electrolyte while in methylbenzene it dissolve in molecular state√ ½
-
- It is the change of the substance from its gaseous state into solid state without going through the liquid state.
- Add dilute hydrochloric acid / sulphuric (VI) acid to the mixture. Zinc reacts to a soluble salt of zinc, while copper doesn’t react. Filter the mixture to obtain the copper powder as the residue
-
- Haematite
- Carbon(II) oxide
Carbon (core…)
-
- Ca(HCO3)2(aq) → CaCO3(s) + CO2(g) + H2O(l)
Mg(HCO3)2(aq) → MgCO3(s) + CO2(g) + H2O(l) - Use of distillation method
Use of ion exchanger
Addition of sodium carbonate solution - Stains white clothes
Reposition of fur(calcium carbonate) in kettles,pipes and boilers hence reducing their efficiency
- Ca(HCO3)2(aq) → CaCO3(s) + CO2(g) + H2O(l)
-
- Diamond and Graphite
- A white precipitate that dissolves to form a colourless solution
Ca(OH)2(aq) + CO2(g) → Ca CO2(s) + H2O(l)
CaCO3(s) + (H2O)2 + CO2(g) → Ca (HCO3(aq))2
- Na2 CO3 xH2O
% mass 36.8 63.2
Molar mass 106 18
No of moles 0.3472 3.5111
0.3472 0.3472
1 10.1
x = 10 -
- The orange lead(II) oxide turned grey
Colourless droplets on the cooler parts - 3PbO(s) + 2NH3(g) → 3Pb(s) + N2(g) + 3H2O(l)
- Used as a raw material in industrial manufacture of nitric(V) acid manufacture of nitrogenous fertilizer
- The orange lead(II) oxide turned grey
-
- Alkaline earth metals
- The atomic radius is bigger than the ionic radius. The resulting positively charged ion experience greater nuclear attraction
-
- They are saturated hence non-sooty
-
-
- Mass of solute that will saturate 100g of water at a certain temperature
-
- 38 x 2 = 76g
- 80-79 = 1g
-
- Copper(II) carbonate
- Effervescence would start and stop. This is because lead(II) chloride which is insoluble would form that coats lead(II) carbonate
-
- Group(VIII) react by gaining electrons thus as the atoms become bigger down the group they have less attraction for electron.
- Moist blue litmus paper turns red and then its bleached. Dry blue litmus paper remained blue.
-
- Zinc and dilute hydrochloric acid/dil sulphuric(vi) acid
-
- unreacted hydrogen
- The solid changed colour from white to blue. This is because when hydrogen reduces copper(II) oxide its oxidized to steam which condenses on the cooler part of the combustion tube and turns the compound blue.
-
- Colourless, characteristic irritating choking smell, denser than air, soluble in water
- Sulphur(IV) oxide reduces dichromate(VI) ions to chromium
- The exhaust gases are passed through a chimney lined with calcium hydroxide which reacts with SO2 scrubbing.
-
-
- Ammonium nitrate
- warm water
- To prevent water that form on the cooler part of flask from flowing to the hot part of the flask.To prevent cracking of the flask by water which forms on the cooler part.
-
-
- Has string metallic bonds with giant metallic structure hence high meeting point
- Has giant covalent structure where atoms are covalently bonded hence no delocalized eletron.
- Both ethanol and water are polar hence ethanol forms hydrogen bonds with water.
-
-
- Purple vapour, due to formation of iodine gas
2I-(l) → 12(g) + 2e- Accept equation
-
-
-
- 2CO(g) + O2(g) → 2CO2(g)
100cm3 50cm3 100cm3
CO2 gas 100cm3 - Oxygen gas 100 – 50 = 50cm3
- 2CO(g) + O2(g) → 2CO2(g)
-
-
-
- Carbon(IV) oxide
- Filtration
- Calcium oxide
- Ammonia
- NH4Cl(aq) + Ca(OH)2(s) → CaCl2(s) + H2O(l) + NH3(g)
-
Download Chemistry Paper 1 Questions and Answers - Asumbi Girls Highschool Pre-Mock Exams May-June 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

