INSTRUCTIONS TO CANDIDATES:
- Answer all the questions in the spaces provided in the question paper.
- You are NOT allowed to start working within the first 15 minutes of the 2 ¼ hours allowed for this paper. This time is to enable you read the question paper and make sure you have all the chemicals and apparatus that you may need.
- All working MUST be clearly shown.
- Mathematical tables and silent scientific calculators may be used.
- Candidates should check to ascertain that all papers are printed as indicated and that no questions are Missing
For Examiner’s Use Only:
|
Question |
Maximum score |
Candidate’s score |
Examiner’s initials |
|
1 |
14 |
||
|
2 |
10 |
||
|
3 |
10 |
||
|
4 |
06 |
||
|
Total score |
40 |

QUESTIONS
- You are provided with:
- Solution A1, potassium iodate solution
- Solution A2, acidified sodium hydrogen sulphite solution
- Solution A3 starch indicator
- Distilled water in a wash bottle.
- Stop watch / stop clock
You are required to find out the effect of concentration of potassium iodate A1 on the rate of reaction with acidified sodium hydrogen sulphite A2.
Note: the end point of reaction of potassium iodate with acidified sodium hydrogen sulphite is indicated in the formation of a blue coloured complex using starch indicator.
Procedure 1:
- Using a 10 cm3 measuring cylinder to pour 5 cm3 of aqueous sodium hydrogen sulphite into the conical flask.
- Use another 10 cm3 of measuring cylinder to pour 5 cm3 of starch solution into the same conical flask.
- Using a burette pour 15 cm3 of distilled water into the same beaker.
- Using a burette pour 20 cm3 of aqueous potassium iodate into the beaker and immediately start the stop watch.
- Swirl the mixture in the conical / flask and continue to swirl until a sudden blue colour change is seen.
- Stop the stop-watch and record time taken seconds for the sudden blue colour change to appear.
- Rinse the beaker with water.
Experiment 2:
- Repeat procedure 1 using 17 cm3 of distilled water and 18 cm3 of aqueous potassium iodate.
- Repeat procedure 1 using 21 cm3 of distilled water and 14cm3 of aqueous potassium iodate.
- Repeat experiment 1 using 23 cm3 of distilled water and 12 cm3 of aqueous potassium iodate.
- Repeat experiment 1 using 25 cm3 of distilled water and 10 cm3 of aqueous potassium iodate.
(a) Complete the table below.
Table I
|
Experiment |
1 |
2 |
3 |
4 |
5 |
|
Volume of Sodium hydrogen sulphite (Na HSO3) used |
5 |
5 |
5 |
5 |
5 |
|
Volume of distilled water used (cm3) |
15 |
17 |
21 |
23 |
25 |
|
Volume of potassium iodate (KIO3 (aq) used in cm3 |
20 |
18 |
14 |
12 |
10 |
|
Time taken to change colour (secs) |
(4 marks)
(b) On the grid below plot a graph of time taken (secs) for the colour change (vertical axis) against volume of aqueous potassium iodate used (cm3). (3 marks)
(c)
- From your graph determine the time taken for the blue colour to appear if 16 cm3 of aqueous potassium iodate was used. (Show clearly on the graph how you worked out your answer). (1 mark)
- Calculate the volume of distilled water required if 16 cm3 of aqueous potassium iodate was used. (1 mark)
(d) On the graph sketch the graph that could be expected if the above experiment s were done at a higher temperature. Explain. (1 mark)
(e) Calculate the concentration of potassium iodate solution in moles per litre in the final reaction mixture in the experiment 1. (2 marks)
(f) How does the concentration of potassium iodate solution A1, affect its rate of reaction with acidified sodium hydrogen sulphite A2? Explain your answer. (2 marks)
2. You are provided with:
- Solution B, which is 0.05M acidified potassium manganate (VII) solution (KMnO4).
- Solution C, containing 5.0g/l of a dibasic acid, H2 2H2O
You are required to:
- Determine the concentration of dibasic acid H2X, solution C and then the formula mass of X.
Procedure II
- Fill the burette with solution B.
- Using a clean pipette, place 25 cm3 of solution C into a clean conical flask. Heat this solution to about 700
- Titrate using solution B until a permanent pink colour just appears. Shake thoroughly during titration.
- Record the reading in table I below.
- Repeat the titration one more time to complete the table below.
Complete the table I below.
Table I
|
I |
II |
|
|
Final burette reading (cm3) |
||
|
Initial burette reading (cm3) |
||
|
Volume of solution b used cm |
(3 marks)
- Determine the average volume of solution B used. (1 mark)
- Calculate:
- The number of moles of manganate (VII) ions in the average volume of solution B used above. (1 mark)
- Given that 2 moles of manganate (VII) ions react with 5 moles of dibasic acid H2 X.2H2O. Calculate the number of moles of the dibasic acid H2 X.2H2O in the 25 cm5 of solution C. (2 marks)
- The concentration of solution C in moles per litre. (1 mark)
- Calculate the formula mass of X in the dibasic acid H2 2H2O (H = 1, O = 16) (2 marks)
3. You are provided with solution Q. Carry out the tests below. Write your observations and inferences in the spaces provided.Place about 2 cm3 of the solution in five separate test-tubes.
a). To the first portion, add aqueous sodium hydroxide drop wise until in excess.
|
Observations |
Inferences |
|
(1 mark) |
(1 mark) |
b). To the second portion, add aqueous ammonia dropwise until in excess.
|
Observations |
Inferences |
|
(1 mark) |
(1 mark) |
c). To the third portion, add 3 drops of dilute hydrochloric acid.
|
Observations |
Inferences |
|
(1 mark) |
(1 mark) |
d). To the fourth portion, add 3 drops of barium nitrate solution.
|
Observations |
Inferences |
|
(1 mark) |
(1 mark) |
e). To the last portion, add 3 drops of lead (II) nitrate solution then warm the mixture.
|
Observations |
Inferences |
|
(1 mark) |
(1 mark) |
4. You are provided with solid R. Carry out the tests below. Write your observations and inferences in the spaces provided.
i). Place one third of solid R on a metallic spatula. Burn it in non-luminous flame of the Bunsen burner.
|
Observations |
Inference |
|
( ½ mark) |
( ½ mark) |
ii). Place the remaining solid in a test-tube. Add about 6 cm3 of distilled water and shake the mixture well. Retain the solution for the next procedure.
|
Observations |
Inferences |
|
( ½ mark) |
( ½ mark) |
I). In another 2 cm3, add 2 drops of acidified potassium manganate (VII).
|
Observations |
Inferences |
|
(1 mark) |
(1 mark) |
(II) To about 1cm3, add 3 drops of acidified potassium dichromate (VI) and warm.
|
Observations |
Inferences |
|
( ½ mark) |
( ½ mark) |
(III) To about 2 cm3 of the solution, add 1g of solid D; sodium hydrogen carbonate.
|
Observations |
Inferences |
|
( ½ mark) |
( ½ mark) |

MARKING SCHEME
- You are provided with:
- Solution A1, potassium iodate solution
- Solution A2, acidified sodium hydrogen sulphite solution
- Solution A3 starch indicator
- Distilled water in a wash bottle.
- Stop watch / stop clock
You are required to find out the effect of concentration of potassium iodate A1 on the rate of reaction with acidified sodium hydrogen sulphite A2.
Note: the end point of reaction of potassium iodate with acidified sodium hydrogen sulphite is indicated in the formation of a blue coloured complex using starch indicator.
Procedure 1:
- Using a 10 cm3 measuring cylinder to pour 5 cm3 of aqueous sodium hydrogen sulphite into the conical flask.
- Use another 10 cm3 of measuring cylinder to pour 5 cm3 of starch solution into the same conical flask.
- Using a burette pour 15 cm3 of distilled water into the same beaker.
- Using a burette pour 20 cm3 of aqueous potassium iodate into the beaker and immediately start the stop watch.
- Swirl the mixture in the conical / flask and continue to swirl until a sudden blue colour change is seen.
- Stop the stop-watch and record time taken seconds for the sudden blue colour change to appear.
- Rinse the beaker with water.
Experiment 2:
- Repeat procedure 1 using 17 cm3 of distilled water and 18 cm3 of aqueous potassium iodate.
- Repeat procedure 1 using 21 cm3 of distilled water and 14cm3 of aqueous potassium iodate.
- Repeat experiment 1 using 23 cm3 of distilled water and 12 cm3 of aqueous potassium iodate.
- Repeat experiment 1 using 25 cm3 of distilled water and 10 cm3 of aqueous potassium iodate.
(a) Complete the table below.
Table I
|
Experiment |
1 |
2 |
3 |
4 |
5 |
|
Volume of Sodium hydrogen sulphite (Na HSO3) used |
5 |
5 |
5 |
5 |
5 |
|
Volume of distilled water used (cm3) |
15 |
17 |
21 |
23 |
25 |
|
Volume of potassium iodate (KIO3 (aq) used in cm3 |
20 |
18 |
14 |
12 |
10 |
|
Time taken to change colour (secs) |
196 | 205 | 240 | 338 | 440 |
(4 marks)
(b) On the grid below plot a graph of time taken (secs) for the colour change (vertical axis) against volume of aqueous potassium iodate used (cm3). (3 marks)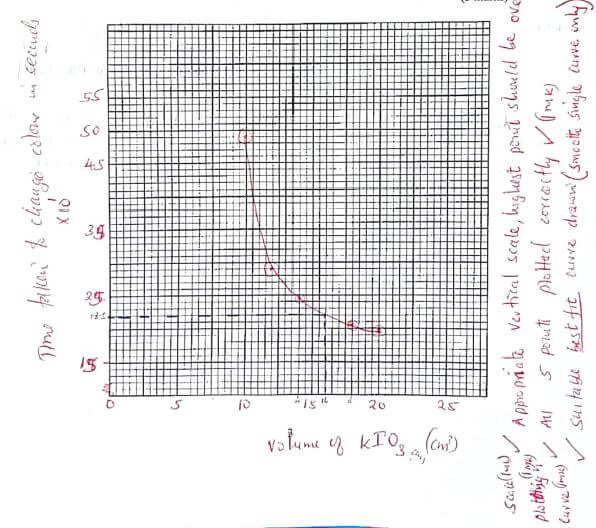
(c)
- From your graph determine the time taken for the blue colour to appear if 16 cm3 of aqueous potassium iodate was used. (Show clearly on the graph how you worked out your answer). (1 mark)
18.5 x 101 = 185 seconds
time correct to their working - Calculate the volume of distilled water required if 16 cm3 of aqueous potassium iodate was used. (1 mark)
35 - 16 = 19cm3
increase in temperature leads to increase in rate of reaction or less time taken to react
(d) On the graph sketch the graph that could be expected if the above experiment s were done at a higher temperature. Explain. (1 mark)
line is below plotted line
(e) Calculate the concentration of potassium iodate solution in moles per litre in the final reaction mixture in the experiment 1. (2 marks)
0.05 → 8 KIO3 - 1000cm3
x → 20cm3
x = 0.05 x 20
1000
= 0.001 moles
0.001 moles KIO3 → 45cm3
x → 1000
x = 0.001 x 1000 = 0.02222M
45
(f) How does the concentration of potassium iodate solution A1, affect its rate of reaction with acidified sodium hydrogen sulphite A2? Explain your answer. (2 marks)
increase in concentration leads to increase in the rate of reaction hence less time to react. The more the concentration (moles) number of particles leads to more collisions hence less time taken to react
2. You are provided with:
- Solution B, which is 0.05M acidified potassium manganate (VII) solution (KMnO4).
- Solution C, containing 5.0g/l of a dibasic acid, H2 2H2O
You are required to:
- Determine the concentration of dibasic acid H2X, solution C and then the formula mass of X.
Procedure II
- Fill the burette with solution B.
- Using a clean pipette, place 25 cm3 of solution C into a clean conical flask. Heat this solution to about 700
- Titrate using solution B until a permanent pink colour just appears. Shake thoroughly during titration.
- Record the reading in table I below.
- Repeat the titration one more time to complete the table below.
Complete the table I below.
Table I
|
I |
II |
|
|
Final burette reading (cm3) |
8.0 | 8.0 |
|
Initial burette reading (cm3) |
0.0 | 0.0 |
|
Volume of solution b used cm |
8.0 | 8.0 |
(3 marks)
- Determine the average volume of solution B used. (1 mark)
8.0 + 8.0 = 16.0 = 8.0cm3 ± 0.2
2 2 - Calculate:
- The number of moles of manganate (VII) ions in the average volume of solution B used above. (1 mark)
0.05 moles → 1000 cm
x → 8 cm
x = 0.05 x 8 = 0.004 moles
1000 - Given that 2 moles of manganate (VII) ions react with 5 moles of dibasic acid H2 X.2H2O. Calculate the number of moles of the dibasic acid H2 X.2H2O in the 25 cm5 of solution C. (2 marks)
MnO4:H+
2:5
0.0004:x
x = 0.0004 x 5
2
= 0.001 moles - The concentration of solution C in moles per litre. (1 mark)
0.001 moles of C → 25 cm3
x → 1000 cm
x = 0.001 x 1000
25
= 0.04m - Calculate the formula mass of X in the dibasic acid H2 2H2O (H = 1, O = 16) (2 marks)
molarity = g/l
R.Fm
0.04 = 5g/l
x
x = 5 ÷ 0.04 = 125
H2A.2H2O = 125
2 + x +2 (18) = 125
x = 125 - 36 - 2
=87
- The number of moles of manganate (VII) ions in the average volume of solution B used above. (1 mark)
3. You are provided with solution Q. Carry out the tests below. Write your observations and inferences in the spaces provided.Place about 2 cm3 of the solution in five separate test-tubes.
a). To the first portion, add aqueous sodium hydroxide drop wise until in excess.
|
Observations |
Inferences |
|
white precipitate soluble in excess |
Zn2+, Al3+, Pb2+ present |
b). To the second portion, add aqueous ammonia dropwise until in excess.
|
Observations |
Inferences |
|
White precipitataae insoluble in excess |
Al3+, Pb2+ present |
c). To the third portion, add 3 drops of dilute hydrochloric acid.
|
Observations |
Inferences |
|
No white precipitate No effervescence |
Al3+ present, Pb2+ absent SO32-, CO32-, HCO3- absent |
d). To the fourth portion, add 3 drops of barium nitrate solution.
|
Observations |
Inferences |
|
No white precipitate |
SO42- absent |
e). To the last portion, add 3 drops of lead (II) nitrate solution then warm the mixture.
|
Observations |
Inferences |
|
White precipitate that dissolves in warming |
Cl- present |
4. You are provided with solid R. Carry out the tests below. Write your observations and inferences in the spaces provided.
i). Place one third of solid R on a metallic spatula. Burn it in non-luminous flame of the Bunsen burner.
|
Observations |
Inference |
|
Burns with a yellow sooty flame |
|
ii). Place the remaining solid in a test-tube. Add about 6 cm3 of distilled water and shake the mixture well. Retain the solution for the next procedure.
|
Observations |
Inferences |
|
dissolves to form a colourless solution homogeneous mixture |
polar compund |
I). In another 2 cm3, add 2 drops of acidified potassium manganate (VII).
|
Observations |
Inferences |
|
acidified potassium manganate (vii) changes colour from purple to colourless |
|
(II) To about 1cm3, add 3 drops of acidified potassium dichromate (VI) and warm.
|
Observations |
Inferences |
|
Yellow acidified potassium dichromate (vi) remains yellow |
R-OH absent |
(III) To about 2 cm3 of the solution, add 1g of solid D; sodium hydrogen carbonate.
|
Observations |
Inferences |
|
Effervesce and bubbles of a colourless gas given out |
O |

CONFIDENTIAL
The information contained in this paper is to enable the head of the school and the teacher in charge of Chemistry to make adequate preparation for the Chemistry Practical Examination.
NO ONE ELSE should have access to this paper or acquire knowledge of its content. Great care MUST be taken to ensure that the information herein does NOT reach the candidates either directly or indirectly. The teacher in charge of Chemistry should NOT perform any of the experiments in the SAME room as the candidates nor make the results of the experiment available to the candidates of give any information related to the experiments to the candidates. Doing so will constitute an examination irregularity.
Requirements for candidates in addition to fittings, and apparatus found in the chemistry laboratory, each candidate will require:
- 100 cm3 of solution A1 – KIO3, potassium iodate solution
- 100 cm3 of solution A2 – Acidified NaHSO3, Acidified sodium hydrogen sulphite
- 150 cm3 of solution B acidified potassium manganite (VII) KmnO4
- 150 cm3 of solution C, 5g/l of dibasic acid, H22H2O
- About 1g of solid R in a stopper container – maleic acid (pure)
- About 1g of solid D (sodium hydrogen carbonate)
- One 50 cm3 burette
- About 30 cm3 of A3, starch indicator solution.
- About 10 cm3 solution Q (Al Cl3 (aq)) in a stoppered boiling tube
- One 25 cm3 pipette
- Two 10 cm3 measuring cylinder
- One 100 cm3 beaker
- Two 250 cm3 conical flasks
- Seven, clear dry test-tubes placed in a rack
- One stop watch / stop clock
- One boiling tube
- One spatula metallic
ACCESS TO:
- 2M sodium hydroxide solution supplied with a dropper.
- 2M Ammonia solution supplied with a dropper
- 2M dilute hydrochloric acid supplied with a dropper.
- 2M Barium nitrate solution supplied with a dropper.
- 2M Lead (II) nitrate solution supplied with a dropper.
- 300 cm2 of distilled water.
- Source of heat (Bunsen burner).
- Acidified potassium manganite (VII) – KmnO4 supplied with a dropper.
- Acidified potassium dichromate(VI) – K2Cr2O7.
PREPARATIONS
- Solution A1 is prepared by dissolving 2g of solid A1 (potassium iodate [KIO3(s)]) in distilled water and making it up to one litre.
- Solution A2 is prepared by dissolving 0.40 g of solid A2 acidified sodium hydrogen sulphite (NaHSO3) in about 200 cm3 of distilled water, and adding 20 cm3 of 1M sulphuric acid, shaking well, and making up to one litre with distilled water.
- Solution A3 – starch indicator is prepared placing 1.0g of solid A3 – starch indicator in 100 cm3 beaker and adding 2 cm3 of distilled water to make a paste and pouring the paste into 100 cm3 of boiling distilled water and boiling the mixture for about one minute and allowing it to cool solution A3 is to be prepared in the morning of the examination.
- Solution B – Acidified potassium manganate(VII) prepared by dissolving 9g of solid potassium manganate(VII) in about 600 cm3 of 2M sulphuric(VI) acid and adding distilled water to make a litre of the solution.
- Solid R is pure maleic acid.
- Solution C – 5g/l of oxalic acid is prepared by dissolving 5 g of oxalic acid in 250 cm3 of water and making it to one litre of solution.
Download Chemistry Paper 3 Questions and Answers - Kassu Jet Pre Mocks 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

