INSTRUCTIONS TO CANDIDATES
- Answer ALL questions
- Mathematical tables and silent electronic calculators may be used.
- All workings MUST be clearly shown where necessary.
For Examiners use only.
|
Question |
Maximum Score |
Candidates Score |
|
|
|
1 – 27 |
80 |

QUESTIONS
- A mixture of magnesium powder and copper powder was reacted with dilute hydrochloric acid.
The solution was then filtered.
Name:-
- The residue (1mk)
- The filtrate (1mk)
- Write an ionic equation for the reaction that takes place (1mk)
-
- Aluminium chloride solution changes the blue litmus paper red. Explain this observation (2mks)
- The diagram below shows the set-up that can be used to prepare and collect oxygen gas. Study it and answer the questions that follow.
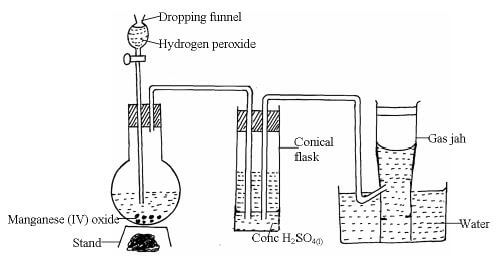
- Identify two mistakes from the diagram which must be corrected for one to collect dry oxygen gas (2mks)
- What property of oxygen gas makes it possible to be collected over water? (1mk)
- The table below gives information on four elements by letters K, L, M and N. Study it and answer the questions that follow. The letters do not represent the actual symbol of the elements.
Element
Electron arrangement
Atomic radius (nm)
Ionic radius (nm)
Q
2.8.2
0.136
0.065
R
2.8.7
0.099
0.181
S
2.8.8.1
0.203
0.133
T
2.8.8.2
0.174
0.099
- Which two elements have similar chemical properties? Explain (2mks)
- What is the most likely formula of the oxide of R? (1mk)
- Which element is a non-metal. Explain (1mk)
- A fixed mass of a gas has a volume of 250cm3 at a temperature of 27ºC and 750mmHg pressure. Calculate the volume the gas would occupy at 42ºC and 750mmHg pressure. (3mks)
- Zinc metal and Hydrochloric acid react according to the following equation
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
1.96g of zinc were reacted with 100cm3 of 0.2M Hydrochloric acid,- Determine the reagent that was not enough (2mks)
- Calculate the total volume of hydrogen gas that was liberated at S.T.P conditions
(Zn = 65.4, molar gas volume = 22.4 litres at S.T.P) (1mks)
-
- Explain how a sample of CH3CH2OH could be distinguished from a sample of CH3COOH by a chemical test (2mks)
- Give the name of the type of compound formed when the (a) above are reacted .(1mk)
- A polymer has the following structure
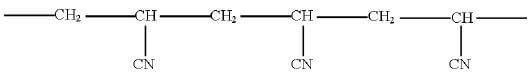
- Draw the repeating unit of the polymer(1mk)
- A sample of this polymer is found to have a molecular mass of 5194. Determine the number of monomers on the polymer (H = 1.0, C = 12.0, N = 14.0) (2mks)
- Describe how the following reagents can be used to prepare lead (II) sulphate:
Solid potassium sulphate, solid lead (II) carbonate, dilute nitric acid and distilled water. (3mks) - Explain why the enthalpy of neutralization of ethanoic acid with sodium hydroxide is different from that of Hydrochloric acid with sodium hydroxide. (3mks)
- Use the information below to answer the questions that follow:
Equation: Enthalpy of formation.- H2(g) + ½ O2(g) H2O(l) ΔH1 = -286kJmol-1
- C(s) + O2(g) CO2(g) ΔH2 = -394kJmol-1
- 2C(s) + 3H2(g) + ½ O2(g) C2H5OH(l) ΔH3 = -277kJmol-1
Calculate the molar enthalpy of combustion of ethanol, given that:
C2H5OH(l) + 3O2(g) 2CO2(g) + 3H2O(l) (3mks)
- The structure shown below represent two cleansing agents A and B.
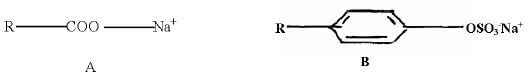
- Which cleansing agent would be more suitable for washing in water containing magnesium sulphate? Explain (2mks)
- Identify the Soapy detergent.(1mk)
- M grammes of a radioactive isotope decayed to 5 grammes in 100 days. The half – life of the isotopes is 25 days.
- What is meant by half – life? (1mks)
- Calculate the initial mass M of the radioactive isotope (2mks)
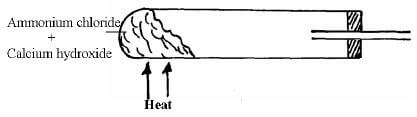
- Complete the diagram to show how a sample of dry ammonia gas can be prepared in the laboratory. (3mks)
- 30cm3 of hydrogen gas was exploded with 10cm3 of oxygen gas at room temperature and pressure. Calculate the total volume of the mixture at;
- 100ºC (2mks)
- 70ºC (1mk)
- Study the scheme below and answer the questions that follow
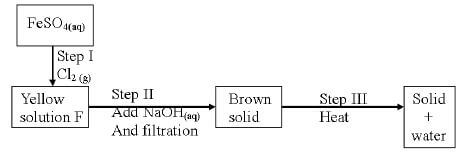
- Write the formula of the cation present in the yellow solution F (1mk)
- What property of chlorine is shown in step I (1mk)
- Write an equation for the reaction in step (III) (1mk)
- A student set up the experiment below to collect gas K. The glass wool was heated before heating the zinc powder.
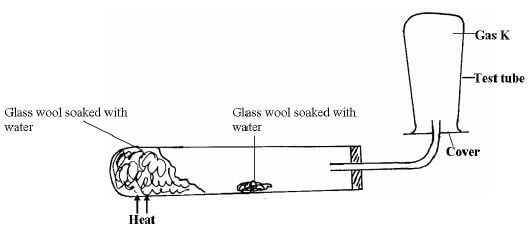
- Why was it necessary to heat the moist glass wool before heating the zinc powder (2mk)
- What observations were made in the test tube (1mk)
- Using dots (•) and crosses (+) to represent the outermost electrons, draw the structure to show the bonding in CO2. (C=6, O = 8). (3mks)
- Calculate the mass of nitrogen(IV)oxide gas that would occupy the same volume as 10g of hydrogen gas at the same temperature and pressure. (H = 1.0, N = 14.0, O = 16.0) (3mks)
- Below is a table of reduction potentials and voltage of some half cells. The letters are not actual symbols but use them to answer the questions which follow
Reaction volts
A2+(aq) + 2e → A(s) -2.80
B+(aq) + e → B(s) -1.50
2C+(aq) + 2e → C2(g) 0.00
D2(g) + 2e → 2D-(aq) +3.20
G+(aq) + e → G(s) +1.80- Select the species with the largest
- Oxidizing power (1mk)
- Reducing power (1mk)
- Calculate the electrode potential (e.m.f) for a cell constructed using half-cells of A and B (1mk)
- Select the species with the largest
- The following table gives the melting points oxides of elements in period 3. Study it and answer the questions that follow:-
Formula of oxide
Na2O
MgO
Al2O3
SiO2
P4O10
SO3
Melting point (0C)
1190
3080
2050
1730
560
-73
- Explain the difference in the melting point of MgO and P4O10 (2mks)
- Name the compound in the above table that will dissolve both in dilute hydrochloric acid and dilute sodium hydroxide (1mk)
- Study the information in the table below and answer the questions that follow
Calculate the enthalpy change of the reactionBond
Bond energy (KJmol-1)
C – H
414
Cl – Cl
244
C – Cl
326
H - Cl
431
CH4(g) + 2Cl2(g) CH2Cl2(g) + 2HCl(g) (3mks) -
- Urea, (NH2)2CO is prepared by the reaction between ammonia and carbon(IV)oxide
2NH3(g) + CO2(g) → (NH2)2CO(aq) + H2O(l)
In one process, 340kg of ammonia were reacted with excess carbon (IV) oxide.
Calculate the moles of urea that were formed. (H = 1.0, C = 12. 0, N = 14.0, O = 16.0) (2mks) - What is the oxidation number of Chromium in Cr2O2-7 (1mk)
- Urea, (NH2)2CO is prepared by the reaction between ammonia and carbon(IV)oxide
- An element P has a relative atomic mass of 88. When a current of 0.5 amperes was passed through the fused chloride of P for 32 minutes and 10 seconds, 0.44g of P were deposited at the cathode. Determine the charge on an ion of P. (1 faraday = 96500 coulombs) (3mks)
- In a neutralization experiment 25cm3 of solution of sodium hydroxide containing 8g per litre was required for complete neutralization of 0.245g of a dibasic acid.
Calculate the relative molecular mass of the acid. (Na = 23.0, O = 16, H = 1) (3mks) -
- Name one drying agent for hydrogen chloride (1mk)
- State and explain the observation that would be made when hydrogen chloride gas is bubbled into a solution of silver nitrate. (2mks)
- In an experiment to study the properties of concentrated nitric acid, a mixture of the acid and wood charcoal was heated in a boiling tube.
- What observations were made? Explain your answer (2mks)
- Write an equation for the reaction that took place in the boiling tube (1mk)
Download Chemistry Paper 1 Questions - Mumias West Pre Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
