Instructions to candidates
- Attempt all questions
- All working must be clearly shown.
- Mathematical tables and non-programmable electronic calculators may be used.
For Examiners use only
|
Question |
Max Score |
Student’s Score |
|
1 |
14 |
|
|
2 |
12 |
|
|
3 |
12 |
|
|
4 |
11 |
|
|
5 |
11 |
|
|
6 |
10 |
|
|
7 |
10 |
|
|
Total |
80 |
QUESTIONS
- Study the flow chart that follows and answer the questions that follow.
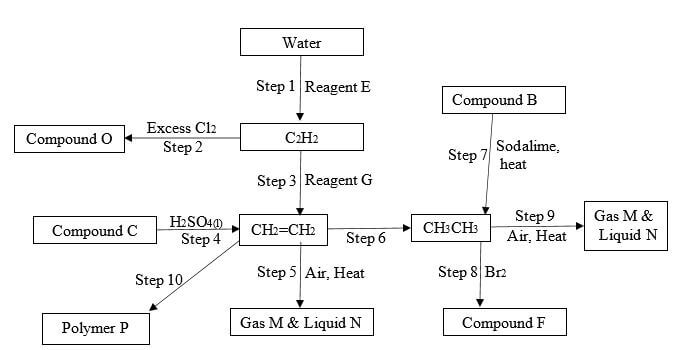
- Name the following 3mrks
- Reagent
E
S - Compound F
- Reagent G
- Compound B
- Compound C
- Compound O
- Reagent
- Name the type of reaction in steps 2, 4. 8 and 9. 2mrks
- step 2
- step 4
- step 8
- step 9
- State any one condition necessary for steps 3, 4, 8 and 10 to take place 2mrks step 3:
step 4:
step 8
step 10 - Draw the structure of compounds P, F and O 3mrks
P:
F:
O: - Steps 5 and 9 are similar and lead to the same products. State and explain one difference in the observations made when steps 5 and 9 are separately carried out. 2mrks
- Write chemical equations for the reactions that take place in steps 2 and 8. 2mrks
Step 2
Step 8
- Name the following 3mrks
-
- Distinguish between an element and a molecule. (2mks)
- The diagram below represents part of the periodic table. Use it to answer the questions that follow. (the letters do not represent the actual symbols of the elements)
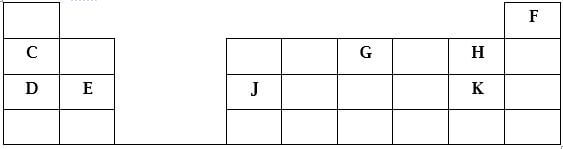
- Which metallic element shown on the table has the highest electrical conductance?
Explain (2mks) - Write the formula of the compound formed between E and G (1mk)
- How do the first ionization energies of elements C and D compare? Explain. (2mks)
- The melting point of E and K are 1120ºC and -34ºC respectively. In terms of structure and bonding, explain why there is a large difference in the melting point of E and K. (2mks)
- Indicate on the grid the position of element L which forms L3- ions with electronic configuration 2.8.8. (1mk)
- Element J reacts with dilute sulphuric(VI)acid at room temperature to produce 0.4dm3 of gas. Determine the mass of J which was reacted with dilute sulphuric(VI)acid. (molar gas volume at rtp is 24dm3, relative atomic mass of J=27) (3mks)
- Which metallic element shown on the table has the highest electrical conductance?
- The graph that follows shows the solubility curves for some three common salts. Study it and answer the questions that follow.
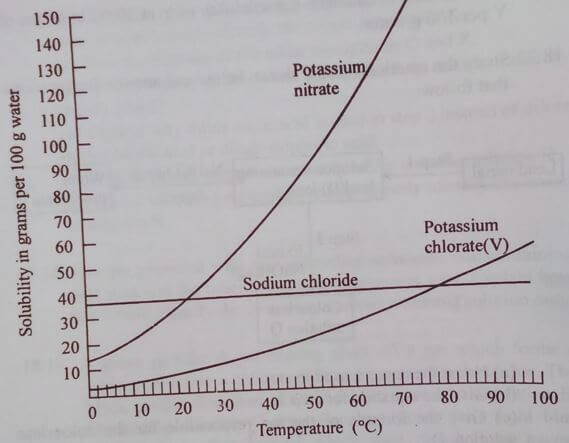
- Which of the three salts has the highest solubility at 20ºC? 1mrk
- Which of the three salts has the lowest solubility at 30ºC? 1mrk
- State the temperature at which the solubility of sodium chloride is the same as that of potassium nitrate and state the solubility of these salts at this temperature. 2mrks
- The solubility of potassium nitrate is 137g/100g of water at 700C and 110g/100g of water at 60ºC. The solubility of potassium chlorate is 32g/100g of water at 700C and 25g/100g of water at 60ºC. Predict what will happen when 100g of water containing 130g of potassium nitrate and 20g of potassium chlorate is cooled from 70ºC to 60ºC. 2mrks
- 100g of water at 60ºC containing 100g of potassium nitrate and 22g of potassium chlorate (v) was cooled to 20ºC. All the crystals formed were filtered.
- Calculate the mass of each salt filtered out. 2mrks
- Determine the composition of the solution at 20ºC after filtration. 2mrks
- Determine the total mass of the solution at 20ºC after filtration. 2mrks
-
- Define the following terms. 3mrks
- Enthalpy of formation.
- Enthalpy of combustion.
- Enthalpy of neutralization
- Study the thermochemical equations below and use them to answer the questions that follow.
- H2(aq)+ ½ O2(g) H2O(l) ΔH= -286kJ/mol
- H+(aq)+ OH-(aq) H2O(l) ΔH= -57.8 kJ/mol
- Give two possible names of the ΔH in b) i) above 2mrks
- Explain why the enthalpy changes above are different yet the equations give the same product. 2mrks
- Use the bond energies that follow to work out the enthalpy changes for the reaction that follows.
CH4(g) + 4Cl2(g) → CCl4(l) + 4HCl(g) 4mrksBond
Bond energy (kJ/mol)
C-H
413
Cl-Cl
242
C-Cl
346
H-Cl
431
- Define the following terms. 3mrks
-
- The diagram that follows represents a set-up that was used to obtain dry nitrogen from the air. Study it and answer the questions that follow
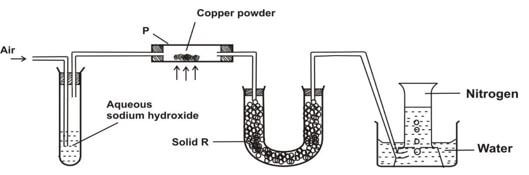
- Name solid R (1mk)
- What is the purpose of sodium hydroxide in the setup? (1mk)
- State and explain the change in mass in tube P at the end of the experiment. (2mks)
- State and explain what would happen to the volume of nitrogen gas collected in the gas jar if magnesium powder was used in place of copper powder (2mks)
- The flow chart below shows the industrial preparation of ammonia and the process used in the manufacture of some ammonium compounds. Study it and answer the question that follows.
- Identify substances S, and V (2mks)
S............................................................
V............................................................ - Name the catalyst and the reagents used in step 6 (2mks)
- State and explain an observation made when conc. Nitric(V)acid is heated with a sample of sulphur. (1mks)
- Identify substances S, and V (2mks)
- The diagram that follows represents a set-up that was used to obtain dry nitrogen from the air. Study it and answer the questions that follow
- The flow chart below represents preparation and properties of oxygen gas. Study it and answer the questions that follow.
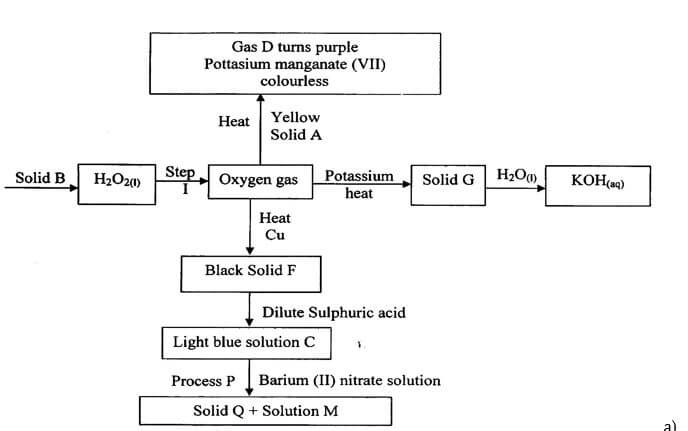
-
- Identify the following substances (2mks)
- Solid A
- Gas D.
- Solid Q.
- Solution M.
- Write a chemical equation for the reaction in step I. (1mk)
- Write chemical equation for the formation of the following compounds. (3mks)
- Solid G.
- Gas D.
- Light blue solution C.
- State the confirmatory test for oxygen gas. (1mk)
- Write the ionic equation for reaction taking place in process P. (1mk)
- State two uses of oxygen. (2mks)
- Identify the following substances (2mks)
-
-
- State Charles’ law 1mk
- Complete the following table by interconverting the following temperatures and filling in the missing volumes assuming that the volumes apply to the same mass of gas at constant pressure. 5mrks
Temperature in 0C
Temperature in K
Volume in litres
195
156
8
312
-234
- A certain gas occupies 700cm3 at -70ºC. If pressure remains constant, at what temperature in ºC will its volume increase by a factor of 3? 4mrks
Download Chemistry Paper 2 Questions - Mumias West Pre Mock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students
