Instructions to candidates
- Answer all the questions
- Non-programmable silent electronic calculators and KNEC mathematical tables may be used.
- All working must be clearly shown where necessary.
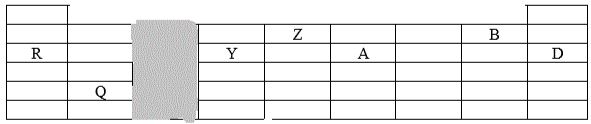
- The grid below represents part of the periodic table. Study it and answer the questions that follow. The letters do not represent the actual symbols of the elements.
- Compare the melting points of R and Y. (2 marks)
- Basing on the structure and bonding, state the structure in; (2 marks)
- Z
- Oxide of Q
- Which element has the lowest ionization energy. (1 mark)
- State the nature of ; (2 marks)
- Oxide of A
- Oxide of Y
- Choose the letter representing an element that; (2 marks)
- Is the most electronegative
- Exist as a monoatomic gas
- Nitrogen falls in the same group as A.
- Write the formula of the compound when A reacts with magnesium. (1 mark)
- Write an equation for the reaction between the compound in f (i) above and water. (1mark)
- State the general property common to elements in the group containing D. Explain. (2 marks)
- Study the table below and use it to answer the questions that follow.
Hydrocarbon Boiling point (K) CH4 112 C2H6 184 C3H8 231 C4H10 273 C5H12 309 C6H14 342 - These organic compounds belong to the same homologous series;
- What is meant by the term homologous series? (1 mark)
- To which homologous series do the above hydrocarbons belong? (1 mark)
- Select one hydrocarbon that would be a liquid at room temperature, 298K. give a reason. (2 marks)
- What is the relationship between the boiling point and the relative molecular masses of the hydrocarbons in the table above? Explain. (2 marks)
- Describe one chemical test used to distinguish between C2H6 and the third member of the homologous series with the general formula CnH2n (2 marks)
- Ethene gas can be prepared in the laboratory by heating ethanol with concentrated sulphuric (vi) acid.
- Name the type of reaction which takes place. (1mark)
- State the temperature required in the reaction b i) above. (1 mark)
- Write the chemical equation for the reaction which produces ethene gas. (1 mark)
- These organic compounds belong to the same homologous series;
-
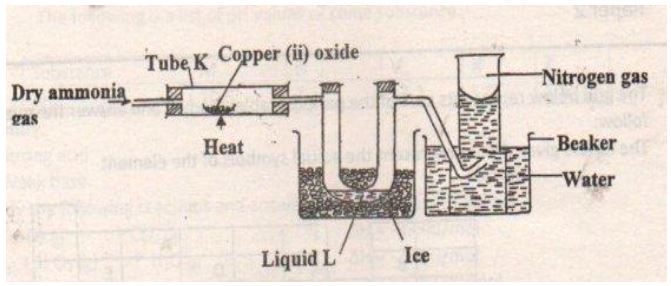
- The diagram below shows the set up that can be used to obtain nitrogen gas in an experiment carried out by form three students.
- How is the ammonia gas for this process dried? (1mark)
- Name liquid L. (1 mark)
- What observation would be made at tube K at the end of the experiment. (1 mark)
- Write an equation for the reaction that took place in the tube K. (1 mark)
- At the end of the experiment the pH of water in the beaker was found to be 10.0 Explain. (1mark)
-
- Ammonia decompose if sparked electrically. What would you expect to be the products of the decomposition? (1 mark)
- Describe the chemical test of ammonia. (2 marks)
- Ammonia gas reacts with water according to the equation below.
NH3(g) + H2O(l) → NH4(aq) + OH−(aq)- Identify the species that acts as a base. Give a reason. (2 marks)
- What effect does addition of sodium hydroxide solution have on the position of the equilibrium? Explain. (2 marks)
- The diagram below shows the set up that can be used to obtain nitrogen gas in an experiment carried out by form three students.
-
- State two factors that should be considered when choosing fuel for cooking. (2 marks)
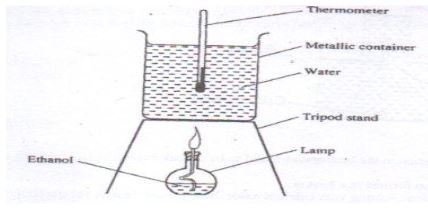
- The diagram below represents a set –up that was used to determine the molar heat of combustion of ethanol
During the experiment, the data given below was recorded
Volume of water 450cm3
Initial temperature of water 25°C
Final temperature of water 46.5°C
Mass of ethanol + Lamp before burning 125.5g
Mass of ethanol + Lamp after burning 124.0g
Calculate the:- Heat evolved during the experiment (density of water=1g/cm3) (2marks)
Specific heat capacity of water =4.2Jg-1K-1 - Molar heat of combustion of ethanol (C = 12.0, O = 16.0, H = 1.0) (2marks)
- Heat evolved during the experiment (density of water=1g/cm3) (2marks)
- Write the thermal chemical equation for the complete combustion of ethanol. (1mark)
- The value of the molar heat of combustion of ethanol obtained (b) (ii) above is lower than the theoretical value. State two sources of error in the experiment. (2 marks)
- Define the term molar heat of combustion (1 mark)
- An impure solid of Copper (II) Carbonate weighing 10.8grams was placed in a beaker containing 50cm3 of dilute Nitric (V) acid. The volume of Carbon (IV) Oxide evolved was recorded at 20 second interval in the table below.
Time from start of reaction (se) 0 20 40 60 80 100 120 Volume of CO2 at S.T.P(litres) 0.0 0.65 0.90 1.07 1.10 1.12 1.12 - Write an equation for the reaction between copper (II) carbonate and nitric (V) acid. (1mark)
- Calculate the reaction between:
- 20 seconds and 40 seconds intervals. (1 mark)
- 40 seconds and 60 seconds intervals. (1 mark)
- Explain the difference in the reaction rates in (b) above. (2marks)
- Why was there no increase in volume of the gas after 100 seconds? (1 mark)
- How many moles of Carbon (IV) oxide were there in the maximum gas produced from this reaction? (M.G.V at S.T.P 22.4 litres). (2 marks)
- What mass of copper (II) carbonate that will have reacted with the acid after 100 seconds? (Cu=64, C=12, O=16) (2 marks)
- Calculate the original concentration of the nitric (V) acid in moles per litre. (2marks)
-
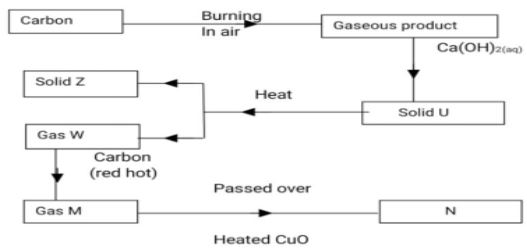
- The diagram below shows several reactions starting with carbon.
- Identify the following U, Z, W, M (2marks)
U
Z
W
M - State the observation made when gas M is passed over heated copper (II) oxide. (1 mark)
- State one agricultural application of solid Z. (1mark)
- Write an equation for the formation of gas M from gas W. (1mark)
- Explain the precaution to be taken in the reaction taking place in (iv) above (1mark)
- Identify the following U, Z, W, M (2marks)
-
- Compare the boiling point of Sulphuric (VI) acid to that of water. Explain (2marks)
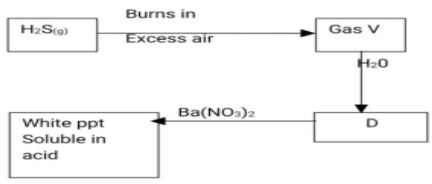
- Study the flow chart below and answer the questions that follow
- Name Gas V,D (1mark)
V
D - Write an ionic equation for the formation of white precipitate. (1mark)
- State the observation made when dilute nitric acid is added to the white precipitate in II above. (1 mark)
- Name Gas V,D (1mark)
- The diagram below shows several reactions starting with carbon.
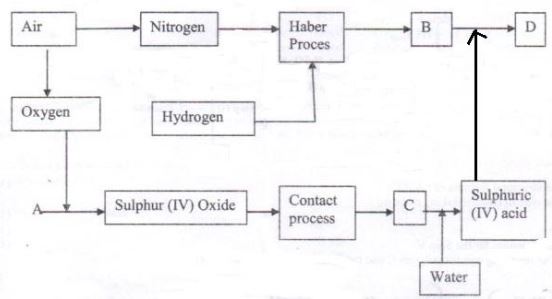
- The flow charts below illustrate two industrial processes, Haber process and the Contact process.
-
- Give the name of the process by which air is separated into oxygen and nitrogen (1mark)
- Apart from oxygen and nitrogen gases produced from process a (i) name one other gas produced. (1 mark)
- Name substances represented by the letters A, B, C (3marks)
A
B
C - Name the catalyst used in:
- Haber process……… (1mark)
- Contact Process………(1mark)
- Explain the role of the catalysts both the Haber process and the Contact processes (1mark)
- Write a chemical equation for the formation of compound B. (1mark)
- Calculate the percentage by mass of the nitrogen present in compound D. (1mark)
- State how compound C is formed. (1mark)
-
MARKING SCHEME
-
- Melting point of Y is higher than that of R. √1Y has a smaller atomic radius which lead to stronger metallic bonds. √1
-
- Giant covalent structure√1
- Giant ionic structure√1
- R√1
-
- Acidic√1
- Amphoteric√1
-
- B√1
- D√11
-
- Mg3A2 or Mg3P2√
- Mg3P2 (s) + 6H2 (l) → 3Mg(OH)2(aq) + 2AH3(g) √1
accept use of actual symbols
- Are inert /do not react. √1Their outermost energy level is completely filled up. √1
-
-
- A group of compounds with similar chemical properties and chemical formula showing a steady gradual change in physical properties. √1
- Alkanes√1
- C5H12 or C6H14. √1 Boiling point is higher than room temperature. √1
- Boiling point increases with increase in molecular mass. √1 Strength of intermolecular forces/ van der Waals forces increases with increase in molecular mass. √1
- Bubble C2H6 and C4H8 through bromine water separately. √1 C4H8 changes bromine water from yellow to colorless while C2H6 does not. √1 OR
Burn C2H6 and C4H8 separately. C2H6 burns with pale blue flame while C4H8 burns with a yellow sooty flame.
-
- Dehydration√1
- Accept any temperature value between 160°C and 180°C√1
- C2H5OH C2H4 + H20 √1 ignore physical states.
-
-
- By passing A trough a U- tube filled with CaO (1mk)
- Water 1mk
- Black solid changes to brown solid (1mk)
- 2NH3(g) +3CUO(s) → 3CU(s) +3H2O(l) +N2(g) (1mk)
- Excess ammonia react with water to form ammonium hydroxide solution which is basic (1mk)
-
- Nitrogen and hydrogen (1mk)
- Dip a glass rod into conc HCL and bring it to the mouth of gas jar full of the gas. (1mk)
Dense white fuse confirms ammonia gas. (1mk)
-
- Ammonia/NH3 1mk It accepts a proton from H2O to form NH4+ 1mk
- Equilibrium shifts to the left√1 concentration of OH- ions which react with NH4+ ions to form NH3 and H2O √1
QUESTION 4
-
- Environmental effect
- Amount of heat produced
- Cost
- Availability
- Ease of storage
-
- 3mks
H = M*C*T 450cm3 * 4.2jg-1 K-1 *(46.5°C − 25°C)
40635 = 40.635kJ
1000 - 2mks
Molar mass CH3 CH2 OH = 46g
Mass of ethanol = 125.5g − 124.0g = 1.5g √½
Moles of CH3 CH2 OH = 1.5g √ = 0.0326moles
46
40.635 ½ = − 12446.4724kJmole-1 ½ (-ve sign)
0.0326
- 3mks
- 1mk
CH3 CH2 OH + 3Oa (g) 2CO2(g) + 3H2O(I) h = −1246.4724kJmol-1 - 1mk
- Heat loss to the surrounding by radiation, conduction, convection.
- Heat absorbed √by reaction vessels.
- Experimental errors √when reading thermometer
- The heat change that occurs when one mole of a substance is completely burnt in oxygen 1mk
QUESTION 5
- CuCO3(s) + 2HNO3(aq) → CU(NO3)2(aq) + CO2(q) + H2O(l)√1
Unbalanced - (0mark)
State symbol missing 1/2mk
0.90 − 0.65 = 0.25
40-20 20 =0.012cm3/sec -
- 1.07 − 0.90 = 0.17
60 − 40 20 = 0.0085cm3
- 1.07 − 0.90 = 0.17
- As time progresses the concentration of reactants reduces. The rate of collision //number of particles colliding reduces hence the rate of reaction √ 1mk decreases
- The reaction has gone into completion //no colliding particles
- 1 mole → 22.4dm3
X ← 1.12dm3
X = 1*1.12 √1mk
22.4
= 0.05 moles√1mk - Moles ratio CUCo3:CO2 = 1:1√1mk
Moles of CUCO3 = 0.05moles√1mk
Mass of = R.F.M *moles
CUCO = 124*0.005√1mk
= 6.2g√1mk - Mole ratio CUCO3:HNO3 = 1:2
Moles of HNO3 acidic = 2*0.05
That reacted = 0.1moles √1mk
50cm contain 0.1moles
1000cm3 X
X = 1000*0.1
50
= 2.0M√1mk
QUESTION 6
-
-
- U-calcium carbonate @√1mk
- Z-calcium oxide
- W-carbon (iv) oxide
- M-carbon (ii) oxide
- Black copper (ii) oxide changesS brown√ 1/2mk
- Liming of soil √1/2mk
- CO2(g) + C(s) → 2CO(g)√1 mk
- Done in the fume chamber or in open√1mk
-
-
- Sulphuric acid has a higher boiling point than water.1mk Sulphuric acid has more number of hydrogen bonds than water √1mk
-
- V-sulphur (iv) oxide √1/2mk
D-sulphurous acid or sulphuric (iv) acid√1/2mk - Ba2+ (aq) + SO32- → BaSO3 (s)
- White precipitate dissolves (1mk)
- V-sulphur (iv) oxide √1/2mk
QUESTION 7
-
- Fractional distillation (1mk)
- Argon (1mk)
-
- A-Sulphur/ ore of sulphur (1mk)
- B- Ammonia gas (1mk)
- C- Oleum (1mk)
- Finely divided iron (1mk)
- Vanadium v oxide/ platinized asbestos (1mk)
- Provide surface area for the reaction and speed up the reaction (1mk)
- N2 (g) + 3H2 (g) → 2NH3(g) (1mk)
- 28/132 x 100 1/2mk
= 21.21% ½ mk - SO3 is bubbled through conc sulphuric acid . (1mk)
This is the last printed page.
Download Chemistry Paper 2 Questions and Answers - Lainaku 1 Joint PreMock Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students