QUESTIONS
- Define the term Chemistry. (1 mk)
- State the major differences between the particles of solids and those of gases. (4 mks)
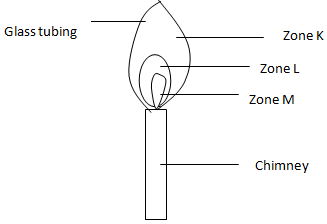
- The diagram alongside shows a non-luminous Bunsen flame (burner). Study it and answer the questions that follow. (3 mks)
- Name the labeled zones based on colour
- J –
- K –
- M –
- Which is the hottest part of the flame? Give a reason for your answer. (2 mks)
- State what would happen if a wooden alighted, splint is placed at the free end of the glass tubing. Explain. (2 mks)
- Why is this flame preferred to a luminous flame for heating purposes? (1 mk)
- Should the air hole be open or closed to produce this flame? Explain.(2 mks)
- A match-stick head placed in zone M will not ignite. Explain. (2 mks)
- Name the labeled zones based on colour
- Give a reason why a candle flame is not suitable for heating in the laboratory. (2 mks)
- Besides a bunsen burner flame, name one other apparatus that can be used conveniently for heating in the laboratory. (1 mk)
- Draw and name 4 common apparatus used in a chemistry laboratory. (4 mks)
- State five laboratory rules observed in a Chemistry laboratory. (5 mks)
- Identify the processes involved in the diagram below. (2 mks)
- A – (½ mk)
- B – (½ mk)
- C – (½ mk)
- D - (½ mk)
- Name one career opportunity in Chemistry. (1 mk)
-
- What is drug abuse? (1 mk)
- What is a drug? (1 mk)
- Explain why most laboratory apparatus are made of glass. (2 mks)
- State four applications of paper chromatography. (4 mks)
- State four characteristics of temperal physical changes (4 mks)
- Define each of the followiing terms
- Atom
- Element
- Compound
- Molecule
- Name the elements present in:-
- Sodium bromide
- Zinc sulphide
- Magnesium nitride
- Potassium iodide

MARKING SCHEME
- Define the term Chemistry. (1 mk)
- It is the systematic study of chemical substances.
- State the major differences between the particles of solids and those of gases. (4 mks)
- Solids have fixed shape while gases have no fixed shape. (2 mks)
- Solids have a fixed volume while gases have no fixed volume.
- The diagram alongside shows a non-luminous Bunsen flame (burner). Study it and answer the questions that follow. (3 mks)
- Name the labeled zones based on colour
- J – Blue zone
- K – Greenish-blue zone
- M – Colourless zone
- Which is the hottest part of the flame? Give a reason for your answer. (2 mks)
- Zone K
- Gas burns completely and rapidly here.
- State what would happen if a wooden alighted, splint is placed at the free end of the glass tubing. Explain. (2 mks)
- Nothing – The gases in zone K completely burns out.
- Why is this flame preferred to a luminous flame for heating purposes? (1 mk)
- It is hot and clean while aluminous flame is not hot enough and is sooty(dirty).
- Should the air hole be open or closed to produce this flame? Explain.(2 mks)
- Open
- This provided entry for air which is necessary for combustion of the air.
- A match-stick head placed in zone M will not ignite. Explain. (2 mks)
- Zone M consists of unburnt gases and is therefore not hot.
- Name the labeled zones based on colour
- Give a reason why a candle flame is not suitable for heating in the laboratory. (2 mks)
- It is not hot enough
- It is sooty
- Besides a bunsen burner flame, name one other apparatus that can be used conveniently for heating in the laboratory. (1 mk)
- Spirit lamp
- Draw and name 4 common apparatus used in a chemistry laboratory. (4 mks)
- State five laboratory rules observed in a Chemistry laboratory. (5 mks)
- Proper dressing
- Switching off all gas outlets/tap when not in use
- Following all the instructions from technicians
- Handling instruments with great care.
- Opening all windows/doors when experiment is on.
- Identify the processes involved in the diagram below. (2 mks)
- A – Melting (½ mk)
- B – Vaporisation/evaporation (½ mk)
- C – Deposition (½ mk)
- D - Sublimation (½ mk)
- Name one career opportunity in Chemistry. (1 mk)
- Medicine
- Pharmacy
- Nursing
-
- What is drug abuse? (1 mk)
- Using a drug for a wrong purpose
- What is a drug? (1 mk)
- Is any substance, natural or manufactured which when used alters the way the body functions.
- What is drug abuse? (1 mk)
- Explain why most laboratory apparatus are made of glass. (2 mks)
- Transparent, can see through
- Do not react with most substances/chemicals
- State four applications of paper chromatography. (4 mks)
- In sports, used to identify banned substances
- In the pharmaceutical industry, to test the purity of drugs
- In food industry, to identify contaminants in food and drinks.
- In cosmetic industry, to identify harmful substances.
- State four characteristics of temperal physical changes. (4 mks)
- Are easily reversible
- No new substance formed
- Mass of substance remains the same
- Not accompanied by heat
- Define each of the following terms: (4 mks)
- Atom – Smallest part of an element that takes part in a chemical reaction.
- Element – Pure substance that cannot be split into simple substance.
- Compound – Pure substance composed of 2 or more elements chemically combine.
- Molecule – Group of elements chemically combined together.
- Name the elements present in:- (4 mks)
- Sodium bromide – Sodium bromide
- Zinc sulphide – Zinc sulphur
- Magnesium nitride – Magnesium Nitrogen
- Potassium iodide – Potassium, Iodine
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Questions and Answers - Form 1 Mid Term 1 Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students