INSTRUCTIONS TO STUDENTS
- Answer all questions in this question paper.
- Define the following terms:
- Atomic Number (1mk)
- Mass Number (1mk)
- The Isotopes (1mk)
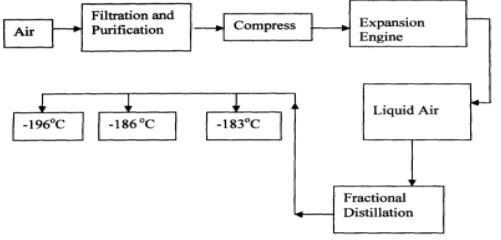
- Oxygen is obtained on large scale by the fractional distillation of air as shown on the flow chart below.
- Explain why air is considered as a mixture (1mk)
- Identify the substance that is removed at the filtration stage (1mk)
- Explain why Carbon (IV) oxide and water are removed before liquefaction of air. (1mk)
- Identify the component that is collected at -186°C (1mk)
- Study the table below and answer the questions that follow:-
Identify with reasons the substances that:Substance
Melting Point (°C )
Boiling point (°C)A B C D E F 801 113 OR 119 − 39 5 − 101 1356 1410 445 457 54 − 36 2860 Electrical
ConductivitySolid Poor Poor Good Poor Poor liquid Good Poor Good Poor Poor - Have a metallic structure (2mks)
- Have a molecular structure (2mks)
- Substances A and C conduct electric current in the liquid state. State how the two substances differ as conductors of electric current (2mks)
- Atoms of element X exists as 14 6 X and 12 6 X
- What name is given to the two types of atoms. (1mk)
- Use dot (∙) and cross (x) diagrams to illustrate the atomic structure of 14 6 X (2mks)
- Give two reasons why most laboratory apparatus are made of glass. (2mk)
- Define the following terms:
- A saturated solution. (1mk)
- Crystallization. (1mk)
- Describe how copper (II) sulphate crystals can be obtained from copper (II) sulphate solution. (3mks)
- Study the table below and use it to answer the questions that follows. Letters are not the actual symbols of the elements
Ion Electronic configuration L− 2,8,8 M2+ 2,8 N3+ 2,8,8 - Which elements belong to the same period of the periodic table? (1 mark)
- What is the formula of the compound formed by L and N.? (1 mark)
- Compare the atomic and ionic radii of element L. (1 mark)
- Write the chemical fomular of the following compounds. 3mks
- Sodium sulphate
- Magnesium hydroxide
- Calcium nitrate.
- State the reasons why carbon ( iv) oxide is used by ice cream venders instead of ordinary ice. (2mks)
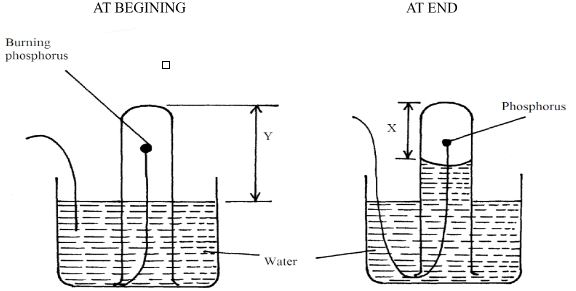
- A student set-up the apparatus below in order to determine the percentage by volume of oxygen in air.
- Why did water rise when the reaction had stopped? (2mks)
- The student wrote the expression for the percentage by volume of oxygen in air as
y − x x 100%
y
Why was the volume of oxygen calculated using the above expression incorrect? (1mk) - What should have been done after the reaction had stopped in order to get a correct volume. (1mk)
- Explain how you would obtain solid lead carbonate from a mixture of lead carbonate and sodium chloride. (3mks)
- Aluminium metal is a good conductor and is used for overhead cables. State any other two properties that make aluminium suitable for this use. (2mks)
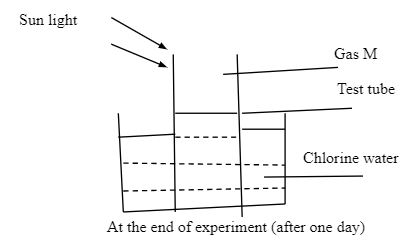
- In an experiment, a test tube of chlorine gas was inverted in water as shown in the diagram. It was then left to stand in sunlight for one day.
After one day, a gas M was found to have collected in the test tube as shown above.- Identify gas M. (1mk)
- Suggest whether the PH of the solution in the beaker would increase or decrease after one day. Give an explanation. (2mks)
- The colour of chlorine water was observed to have changed from pale yellow to colourless after one day. Explain. (2mks)
- Write an equation to support your answer in (iii) above. (1mk)
- State and explain the observation made when a moist blue litmus paper was placed at the mouth of the test tube containing chlorine gas. (3mks)
- Write an equation to show how the process in (v) above occurs. (1mk)
- Give two uses of chlorine gas. (2mks)
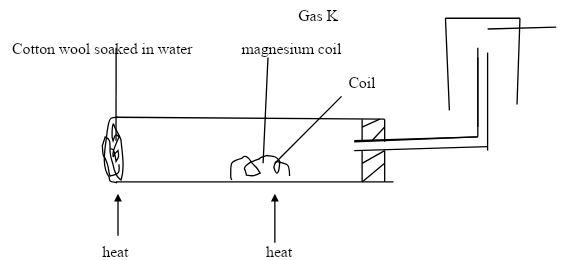
- A student set up the experiment below to collect gas K. the glass wool was heated before heating the magnesium coil.
- Explain why it was necessary to heat the moist cotton wool before heating the magnesium. (2mks)
- Identify gas K. (1mk)
- What property of gas K makes it possible to be collected by the method shown? (1mk)
- Write a chemical equation for the reaction that produced gas K. (1mk)
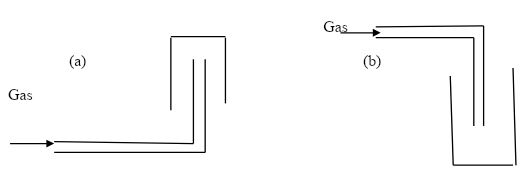
- The diagram represents two methods of gas collection in the laboratory.
- Name the methods of gas collection above. (2mks)
- Which method is suitable for collecting dry carbon (IV) oxide gas? Give a reason. (2mks)
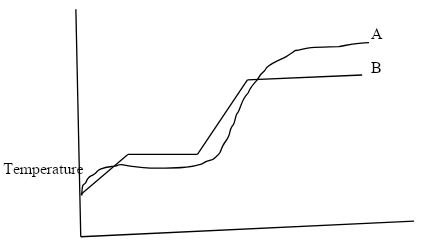
- The curves bellow represent the variation of temperature with time when pure and impure samples of a solid were heated separately.
- Which curve represents the variation in temperature for pure solid? Explain. (2mks)
- State the effect of an impurity on the melting and boiling points of a pure substance. (2mks)
-
- Cars in Mombasa are found to rust faster than cars in Nairobi. Explain. (2 mks)
- State one disadvantage of rusting. (1mk)
- The PH of a soil sample in a given area was found to be 5.5. An Agricultural officer the addition of lime (calcium oxide). State the function of lime in the soil. (1mk)
- By use of dot (.) and cross (x) diagram show bounding in magnesium chloride (mgCl2) (2mks)
MARKING SCHEME
- Define the following terms:
- Atomic Number (1mk)
- No. of protons in the nucleus of an atom
- Mass Number (1mk)
- Sum of proton and neutrons in the nucleus
- The Isotopes (1mk)
- Element with same atomic no. but different mass number
- Atomic Number (1mk)
- Oxygen is obtained on large scale by the fractional distillation of air as shown on the flow chart below.
- Explain why air is considered as a mixture (1mk)
- Various components can be separated using a physical means / method.
- Components in air are not in fixed proportions.
- It contains several gases which are not chemically combined
- Identify the substance that is removed at the filtration stage (1mk)
- Dust particles ✔1
- Explain why Carbon (IV) oxide and water are removed before liquefaction of air. (1mk)
- They would readily solidify✔ ½ and block the pipes ✔ ½
- Identify the component that is collected at -186°C (1mk)
- Argon ✔1
- Explain why air is considered as a mixture (1mk)
- Study the table below and answer the questions that follow:-
Identify with reasons the substances that:Substance
Melting Point (°C )
Boiling point (°C)A B C D E F 801 113 OR 119 − 39 5 − 101 1356 1410 445 457 54 − 36 2860 Electrical
ConductivitySolid Poor Poor Good Poor Poor liquid Good Poor Good Poor Poor - Have a metallic structure (2mks)
- C ✔1 Good conductor of electricity ✔1 in both solid and liquid state due to delocalized
- Have a molecular structure (2mks)
- D or E ✔1 Are poor conducts in both solid / liquid state.
Have relatively low M.P and B.P due to molecular structure.
- D or E ✔1 Are poor conducts in both solid / liquid state.
- Substances A and C conduct electric current in the liquid state. State how the two substances differ as conductors of electric current (2mks)
- A – mobile/free ions
- B – Delocalized electrons
- Have a metallic structure (2mks)
- Atoms of element X exists as 14 6 X and 12 6 X
- What name is given to the two types of atoms? (1mk)
- isotopes
- Use dot (∙) and cross (x) diagrams to illustrate the atomic structure of 14 6 X (2mks)
- What name is given to the two types of atoms? (1mk)
- Give 2 reasons why most laboratory apparatus are made of glass. (2mk)
- Glass can be used for heating
- Glass cannot react with chemicals
- Define the following terms:
- A saturated solution. (1mk)
- A solution that cannot take any more solute at any given temperature
- Crystallization. (1mk)
- Formation of crystals from a saturated solution
- A saturated solution. (1mk)
- Describe how copper (II) sulphate crystals can be obtained from copper (II) sulphate solution. (3mks)
- Heat copper (ii)sulphate solution to evaporate excess water /to obtain a saturated solution
- Cool the saturated solution to obtain copper (ii) sulphate crystals.
- Dry the crystals between filter papers.
- Study the table below and use it to answer the questions that follows. Letters are not the actual symbols of the elements
Ion Electronic configuration L− 2,8,8 M2+ 2,8 N3+ 2,8,8 - Which elements belong to the same period of the periodic table? (1 mark)
- L and M
- What is the formula of the compound formed by L and N.? (1 mark)
- NL3
- Compare the atomic and ionic radii of element L. (1 mark)
- The ion of L has a larger radius than the atom of L.
- Which elements belong to the same period of the periodic table? (1 mark)
- Wright the correct formula of the following compounds. 3mks
- Sodium sulphate
- Na2SO4
- Magnesium hydroxide
- Mg (OH)2
- Calcium nitrate.
- Ca (NO3)2
- Sodium sulphate
- State the reason why carbon ( iv) oxide is used by ice cream venders instead of ordinary ice. (1mks)
- Dry carbon (IV) oxide evaporates leaving no wetness.
- Carbon (IV) oxide is a better coolant
- A student set-up the apparatus below in order to determine the percentage by volume of oxygen in air.
- Why did water rise when the reaction had stopped? (1mk)
- To occupy the space that was initially occupied by oxygen gas
- The student wrote the expression for the percentage by volume of oxygen in air as
y − x x 100%
y
Why the volume of oxygen was calculated using the above expression incorrect? (1mk)- Because oxides of phosphorous formed still occupy space enviously occupied by oxygen.
(P2O5, P2O3) ✔01
- Because oxides of phosphorous formed still occupy space enviously occupied by oxygen.
- What should have been done after the reaction had stopped in order to get a correct volume. (1mk)
- Let all the fumes dissolve in water before final reading is taken ✔01
- Why did water rise when the reaction had stopped? (1mk)
- Explain how you would obtain solid lead carbonate from a mixture of lead carbonate and sodium chloride. (3mks)
- Add water to the mixture and stir to dissolve sodium chloride
- Filter to obtain sodium chloride as a filtrate and lead carbonate as a residue
- Wash the residue and dry it between filter paper
- Aluminium metal is a good conductor and is used for overhead cables. State any other two properties that make aluminium suitable for this use. (2mks)
- Al does not rust
- Al has more delocalized electrons hence a better conductor of electricity.
- In an experiment, a test tube of chlorine gas was inverted in water as shown in the diagram. It was then left to stand in sunlight for one day.
After one day, a gas M was found to have collected in the test tube as shown above.- identify gas M. (1mk)
- Oxygen gas
- Suggest whether the PH of the solution the beaker would increase or decrease after one day. Give an explanation. (2nks)
- PH would decrease.
- Chloric (i) acid (unstable) decompose to hydrochloric acid, which is a strong acid.
- The colour of chlorine water was observed to have changed from pale yellow to colourless after one day. Explain. (2mks)
- Chloric (i) acid is yellow in colour. When exposed to sun light it decomposes to HCl acid and oxygen gas. HCl acid is colourless.
- Write an equation to support your answer in (iii) above. (1mk)
- 2HOCl → 2 HCl (aq) +O2 (g)
- State and explain the observation made when a moist blue litmus paper was placed at the mouth of the test tube containing chlorine gas. (3mks)
- The litmus paper turned red then white. It turned red because of the presence of hydrogen ions then white/ breached by chloric (i) acid though oxidation.
- Write an equation to show how the process in 3(v) above occurs. (1mk)
- HClO(aq) + Dye HCl (aq) + (dye + O)
(coloured) colourless
- HClO(aq) + Dye HCl (aq) + (dye + O)
- Give two uses of chlorine gas. (2mks)
- Used in the manufacture of hydrochloric acid
- Used in making breaches
- Used to make plastics (pvc)
- Used to kill microorganism in water treatment
- identify gas M. (1mk)
- A student set up the experiment bellow to collect gas K. the glass wool was heated before heating the magnesium coil.
- Explain why it was necessary to heat the moist cotton wool before heating the magnesium. (2mks)
- To produce steam this will react with magnesium. Heating magnesium first will make magnesium to react with oxygen.
- Identify gas K. (1mk)
- Hydrogen gas
- What property of gas K makes it possible to be collected by the method shown? (1mk)
- It’s lighter than air
- Write a chemical equation for the reaction that produced gas K. (1mk)
- Mg +H2O → MgO + H2
- Explain why it was necessary to heat the moist cotton wool before heating the magnesium. (2mks)
- The diagram represents two methods of gas collection in the laboratory.
- Name the methods of gas collection above. (2mks)
- Upward delivery
- Downward delivery
- Which method is suitable for collecting dry carbon (IV) oxide gas? Give a reason. (2mks)
- Downward delivery
- Carbon (iv) oxide is denser than air.
- Name the methods of gas collection above. (2mks)
- The curves bellow represent the variation of temperature with time when pure and impure samples of a solid were heated separately.
- Which curve represents the variation in temperature for pure solid? Explain. (2mks)
- B, has sharp melting and boiling point
- State the effect of an impurity on the melting and boiling points of a pure substance.(2mks)
- Impurity lowers the melting point and raises the boiling point
- Which curve represents the variation in temperature for pure solid? Explain. (2mks)
-
- Cars in Mombasa are found to rust faster than cars in Nairobi. Explain. (2mks)
- Mombasa is salty. Salt accelerates rusting.
- State one disadvantage of rusting. (1mk)
- Causes wear and tare.
- Cars in Mombasa are found to rust faster than cars in Nairobi. Explain. (2mks)
- The PH of a soil sample in a given area was found to be 5.5. An Agricultural officer the addition of lime (calcium oxide). State the function of lime in the soil. (1mk)
- Neutralizes the soil.
- Adds calcium to the soil.
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Questions and Answers - Form 2 Mid Term 3 Exams 2023.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students