Questions
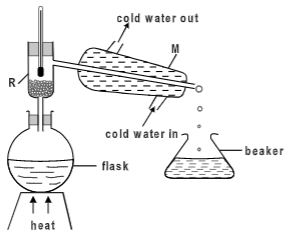
- A student separated liquid P (B.P 78°C) and liquid Q (B.P 100°C) wring the apparatus shown below.
- Name the apparatus labelled
- M (1 mark)
- R (1 mark)
- State one function of the glass bead in apparatus labelled R (1 mark)
- What is the reading on the thermometer when the first jar drops of the distillate appeared in the beaker.(1 mark)
- Which of the liquids remains in the flask.(1 mark)
- Name the apparatus labelled
- Name a method that can be used to extract the following:-
- Common salt from a salt solution.(1 mark)
- Paraffin from crude oil.(1 mark)
- A compound of carbon, hydrogen and oxygen contain 54.55% carbon, 9.09% and remaining 36.36% oxygen. If its relative molecular mass is 88, determine its molecular formula(C=12.0, H =1.0, O= 16.0) [4mark]
- Sodium nitrate(V)can also be used to prepare nitric(V)acid. State two reasons why potassium nitrate(V) is preferred over Sodium nitrate(V). (2marks)
- The atomic number of an element A is 11 and that of B to 8.
- Write down a possible formula of compounded formed between A and B(1mark)
- Draw a dot (•) and cross (×) diagram to show bonding in compound farmed. (2 marks)
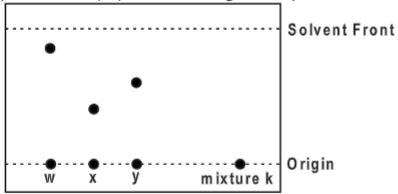
- Below is a paper chromatogram of pure substances W, X and Y
- The mixture K contains substances W and X only. Indicate on the diagram the chromatogram of K.(2 marks)
- State one application of chromatography.(1 mark)
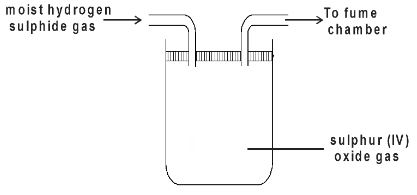
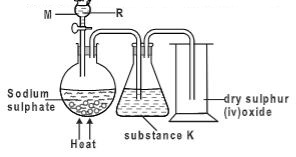
- Moist hydrogen sulphide gas was passed through a tube containing wet sulphur (IV) oxide gas as shown below.
- State the observation (s) made.(1 mark)
- Write an equation for the reaction above.(1 mark)
- Giving a reason, which substance undergoes reduction above.(1 mark)
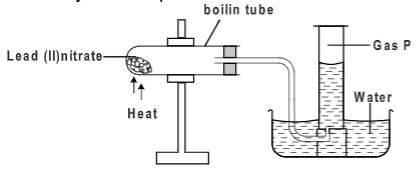
- Study the set up below and use it to answer the questions that follow.
- What observations are made in the boiling tube. Explain.(1 mark)
- Write an equation of reaction occurring in the boiling tube.(1 mark)
- When excess dilute hydrochloric acid was added to sodium sulphate, 960cm³ of sulphuric (IV) oxide gas was produced. Calculate the mass of sodium sulphite that was used. (Molar mass of sodium sulphite = 126g) and molar gas volume at rtp is 24dm³.(3 marks)
- The table below shows atomic and ionic radii of some elements represented by letters U, V, W, X (Not the actual symbols) Study it and answer the questions that follow.
Element Atomic Radius(nm) Ionic radius (nm) U 0.174 0.099 V 0.203 0.133 W 0.099 0.181 X 0.136 0.065 - Classify element X as a metal or non-metal. Explain. (1 mark)
- Which of the elements is the strongest reducing agent? (1 mark)
- Which element forms an anion.(1 mark)
-
- State Graham’s law of diffusion.(1 mark)
- 400cm3 of gas D diffuses from porous plug in 50 seconds while 600cm3 of oxygen diffuses from the same porous plant in 30 seconds. Calculate the relative molecular mass of gas. (O = 16)(3 marks)
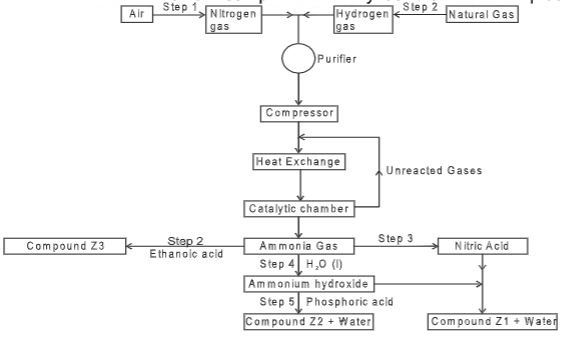
- The flow chart below shows the industrial preparation of ammonia and process used in the manufacture of ammonium compounds. Study it and answer the questions that follow.
- Give the name of the:
- Process in step 1(1 mark)
- Reaction that takes place in step 5 (1 mark)
-
- State one other source of hydrogen gas apart from natural gas. (1 mark)
- Explain why it is necessary to compress nitrogen and hydrogen in this process.(2 marks)
-
- Write an equation for the reaction which takes place in step 2(1 mark)
- Name the catalyst and the reagents used in step 3.
- Catalyst (1 mark)
- Reagents (1 mark)
- Name compound Z1(1 mark)
- Give one commercial use of compound Z2(1 mark)
- Give the name of the:
- What property of concentrated sulphuric (VI) acid is displayed in the following reactions.
- Concentrated sulphuric (VI) acid taking water from gases leaving them dry.(1 mark)
- Concentred sulphuric acid takes water from blue crystals or hydrated copper (II) sulphate, leaving white anhydrous copper (II) sulphate.(1 mark)
- Hot concentrated sulphuric (VI) acid reacts with copper turnings forming copper (II) sulphate sulphur (IV) oxide and water.(1 mark)
- The diagram below shows a set-up that was used to prepare and collect sulphur (IV) oxide gas. Study it and answer the questions that follows.
-
- Name substance R.(1 mark)
- Name apparatus M.(1 mark)
- Write a balanced equation for the reaction between R and Sodium sulphite. (1 mark)
- Why is sulphur (IV) oxide not collected by over water methods.(1 mark)
-
- Identify substance K.(1 mark)
- What is the function of substance K. (1 mark)
-
- The diagram below represents pipes used in the Frasch pump for the extraction of sulphur.
Which substances pass through tubes- 1(1 mark)
- 2 (1 mark)
- 3 (1 mark)
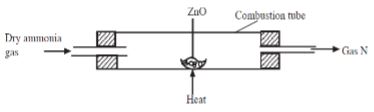
- Dry ammonia gas was passed over hot zinc oxide as shown in the diagram below
- Identify gas N.(1mark)
- State observation made in the combustion tube.(2marks)
- Name the reagents required to produce ammonia gas(2marks)
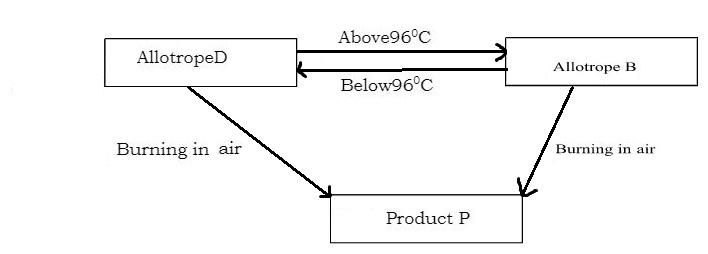
- The flow chart below shows properties of two allotropes of element Q.
- Identify the allotropes:
- D (1mk)
- B (1mk)
- Name element Q(1mk)
- Write a chemical equation for the reaction forming product P.(1mk)
- What term is given to the temperature of 960C shown above?(*1mark)
- Identify the allotropes:
-
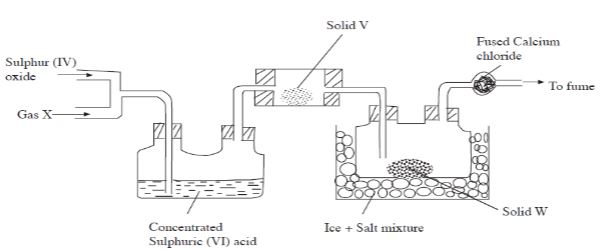
- Name substance: X and Y( 2 mks)
- What is the role of the following substances ?
- Solid V(1 mk)
- Fused calcium chloride(1mk)
- Salt in the Ice + salt mixture(1mk)
- Explain why the fume chamber is used?(1mk)
- Write an equation for the reaction that took place in the combustion tube.(1mk)
- Starting with zinc metal, describe how a solid sample of zinc hydroxide can prepared.(3 marks)
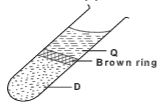
- The substances and apparatus below were used to test the presence of nitrate in substance D.
- Identify substance D (1 mark)
- What are the components of the brown ring.(1 mark)
- Nitrogen does not support combustion yet burning magnesium introduced into a gas jar of nitrogen continues to burn, forming a white solid. Explain.(1 mark)
- Write an equation for the reaction forming the white solid.(1 mark)
- State two uses of nitrogen.(1 mark)
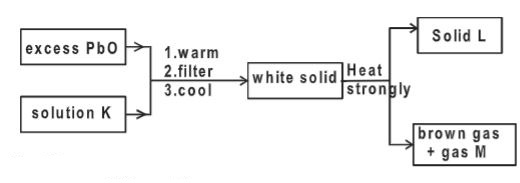
- Study the flow chart below and answer the questions that follow.
Identify- Solution K.(1 mark)
- Solid L(1 mark)
- gas M ( 1 mk)
- Study the scheme below and answer the questions that follow.
Explain the observations made in- Step 1 (1 mark)
- Step 2 (1 mark)
- Step 3 (1 mark)
- Study the diagram below and use it to answer the questions that follow.
- Identify electrodes.(2 marks)
- Name the product formed at the anode.(1 mark)
- The flow diagram below is a summary of the industrial manufacture of sulphuric (VI) acid.
- Write an equation for the reaction in the burner.(1 mark)
- Why is it important to pass gas T and air through cleaners?(1 mark)
- Identify
- Gas U (½ mark)
- Liquid K(½ mark)
- Liquid L(½ mark)
- Write equation for the reaction taking place in the catalytic chamber (1 mark)
- Name the most suitable catalyst that can be used in the catalytic chamber. (1 mark)
- Give the name of the product formed in the absorption tower.(1 mark)
- Write equation for the reaction taking place in the dilution chamber. (1 mark)
- Name the main pollutant in this process and state how it is taken care of.(1½ marks)
- Give one use of sulphuric (VI) acid.(1 mark)
Marking Scheme
- A student separated liquid P (B.P 78°C) and liquid Q (B.P 100°C) wring the apparatus shown below
- Name the apparatus labelled
- M (1 mark)
- liebig condenser
- R (1 mark)
- Fractionating column
- M (1 mark)
- State one function of the glass bead in apparatus labelled R (1 mark)
- To increase surface area for condensation
- What is the reading on the thermometer when the first jar drops of the distillate appeared in the beaker.(1 mark)
- 78
- Which of the liquids remains in the flask.(1 mark)
- water
- Name the apparatus labelled
- Name a method that can be used to extract the following:-
- Common salt from a salt solution.(1 mark)
- evaporation
- Paraffin from crude oil.(1 mark)
- Fractional distillation
- Common salt from a salt solution.(1 mark)
- A compound of carbon, hydrogen and oxygen contain 54.55% carbon, 9.09% and remaining 36.36% oxygen. If its relative molecular mass is 88, determine its molecular formula(C=12.0, H =1.0, O= 16.0) [4mark]
- Empirical formula is C2H4O
The molecular formula is thus determined :
n = Relative formular mass = 88 = 2
Relative empirical formula 44
The molecular formula is (C2H4O ) x 2 = C4H8O2.
- Empirical formula is C2H4O
- Sodium nitrate(V)can also be used to prepare nitric(V)acid. State two reasons why potassium nitrate(V) is preferred over Sodium nitrate(V). (2marks)
- Potassium nitrate(V) is more volatile than sodium nitrate(V) and therefore readily displaced from the less volatile concentrated sulphuric(VI)acid
- Sodium nitrate(V) is hygroscopic and thus absorb water . Concentrated sulphuric(VI)acid dissolves in water. The dissolution is a highly exothermic process
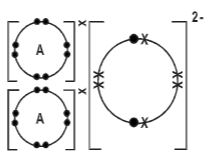
- The atomic number of an element A is 11 and that of B to 8.
- Write down a possible formula of compounded formed between A and B(1mark)
- A2B
- Draw a dot (•) and cross (×) diagram to show bonding in compound farmed. (2 marks)
- Write down a possible formula of compounded formed between A and B(1mark)
-
- Below is a paper chromatogram of pure substances W, X and Y
The mixture K contains substances W and X only. Indicate on the diagram the chromatogram of K.(2 marks)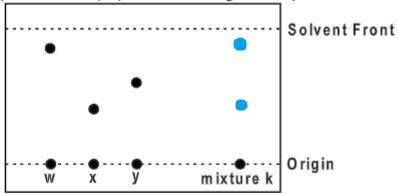
- State one application of chromatography.(1 mark)
- Testing of illegal drugs in urine
- Below is a paper chromatogram of pure substances W, X and Y
- Moist hydrogen sulphide gas was passed through a tube containing wet sulphur (IV) oxide gas as shown below.
- State the observation (s) made.(1 mark)
- Yellow deposit formed
- Write an equation for the reaction above.(1 mark)
- 2H2S(g) + SO2(g) → 3S(s) + 2H2O(l)
- Giving a reason, which substance undergoes reduction above.(1 mark)
- Sulphur (IV) oxide gas is reduced since it accepts electrons from hydrogen sulphide
- State the observation (s) made.(1 mark)
- Study the set up below and use it to answer the questions that follow.
- What observations are made in the boiling tube. Explain.(1 mark)
- Red residue cooled to a yellow residue
- Brown fumes produced
- Write an equation of reaction occurring in the boiling tube.(1 mark)
heat
- 2Pb(NO3)2 → 2PbO(s) + 4NO2(g) + O2(g)
- What observations are made in the boiling tube. Explain.(1 mark)
- When excess dilute hydrochloric acid was added to sodium sulphate, 960cm³ of sulphuric (IV) oxide gas was produced. Calculate the mass of sodium sulphite that was used. (Molar mass of sodium sulphite = 126g) and molar gas volume at rtp is 24dm³.(3 marks)
- Na2SO3(s) + 2HCl(aq) → 2NaCl(aq) + SO2(s) + H2O(l)
1 mole Mole
Mole ratio 1 : 1 ✓½
Moles of Na2SO3 = 0.04 ü½
RMM of Na2SO3 = 126
Mass of Na2SO3 = 0.04 × 126 ½ = 5.04 g
- Na2SO3(s) + 2HCl(aq) → 2NaCl(aq) + SO2(s) + H2O(l)
- The table below shows atomic and ionic radii of some elements represented by letters U, V, W, X (Not the actual symbols) Study it and answer the questions that follow
- Classify element X as a metal or non-metal. Explain. (1 mark)
- Its a metal½ since atomic radius is greater✓½ than ionic radius
- Which of the elements is the strongest reducing agent? (1 mark)
- V
- Which element forms an anion.(1 mark)
- W
- Classify element X as a metal or non-metal. Explain. (1 mark)
-
- State Graham’s law of diffusion.(1 mark)
- The rate of diffusion of a gas is inversely proportional to the square root of its density provided the initial conditions remain constant
- 400cm3 of gas D diffuses from porous plug in 50 seconds while 600cm3 of oxygen diffuses from the same porous plant in 30 seconds. Calculate the relative molecular mass of gas. (O = 16)(3 marks)
- 400/50 = √(32/MMD)
600/30
8/20 = √(32/MMD)
MMD = 32 x 400
64
MMD = 200
- 400/50 = √(32/MMD)
- State Graham’s law of diffusion.(1 mark)
- The flow chart below shows the industrial preparation of ammonia and process used in the manufacture of ammonium compounds. Study it and answer the questions that follow.
- Give the name of the:
- Process in step 1(1 mark)
- Fractional distillation
- Reaction that takes place in step 5 (1 mark)
- neutralization
- Process in step 1(1 mark)
-
- State one other source of hydrogen gas apart from natural gas. (1 mark)
- Cracking of long chain alkanes
- Explain why it is necessary to compress nitrogen and hydrogen in this process. (2 marks)
- High pressure brings the molecules closer/increasing the concentration of gas molecules/the pressure shifts the equilibrium the right; Hence the yield of ammonia increases
- State one other source of hydrogen gas apart from natural gas. (1 mark)
-
- Write an equation for the reaction which takes place in step 2(1 mark)
- NH3(g) + CH3COOH(aq) → CH3CHOONH4
- Name the catalyst and the reagents used in step 3.
- Catalyst- Platinum rhodium /platinum (1 mark)
- Reagents Water and oxygen (1 mark)
- Name compound Z1(1 mark)
- Ammonium nitrate; rej Formula
- Give one commercial use of compound Z2 (1 mark)
- as fertilizer; rej manufacture of fertilizer; 1
- Write an equation for the reaction which takes place in step 2(1 mark)
- Give the name of the:
- What property of concentrated sulphuric (VI) acid is displayed in the following reactions.
- Concentrated sulphuric (VI) acid taking water from gases leaving them dry.(1 mark)
- It is hygroscopic
- Concentred sulphuric acid takes water from blue crystals or hydrated copper (II) sulphate, leaving white anhydrous copper (II) sulphate.(1 mark)
- Dehydrating property
- Hot concentrated sulphuric (VI) acid reacts with copper turnings forming copper (II) sulphate sulphur (IV) oxide and water.(1 mark)
- Oxidising property
- Concentrated sulphuric (VI) acid taking water from gases leaving them dry.(1 mark)
- The diagram below shows a set-up that was used to prepare and collect sulphur (IV) oxide gas. Study it and answer the questions that follows.
-
- Name substance R.(1 mark)
- dilute hydrochloric acid.
- Name apparatus M.(1 mark)
- dropping funnel
- Write a balanced equation for the reaction between R and Sodium sulphite. (1 mark)
- Na2SO3(s) + 2HCl(aq) → 2NaCl(aq) + SO2(g) + H2O(l)
- Why is sulphur (IV) oxide not collected by over water methods.(1 mark)
- It is soluble in water
- Name substance R.(1 mark)
-
- Identify substance K.(1 mark)
- Concentrated sulphuric (VI) acid
- What is the function of substance K. (1 mark)
- To dry sulphur (IV) oxide gas
- Identify substance K.(1 mark)
-
- The diagram below represents pipes used in the Frasch pump for the extraction of sulphur.
Which substances pass through tubes- 1 Compressed hot air in 1
- 2 - Molten froth of sulphur water mixture out
- 3- superheated water-in 1
- Dry ammonia gas was passed over hot zinc oxide as shown in the diagram below
- Identify gas N.(1mark)
- nitrogen gas
- State observation made in the combustion tube.(2marks)
- color of zinc oxide changes from yellow to gey
- colourless liquid formed on cooler parts of the testtube
- Name the reagents required to produce ammonia gas(2marks)
- ammonium chloride and calcium hydroxide
- Identify gas N.(1mark)
- The flow chart below shows properties of two allotropes of element Q.
- Identify the allotropes:
- D rhombic (1mk)
- B monoclinic (1mk)
- Name element Q(1mk)
- sulphur
- Write a chemical equation for the reaction forming product P.(1mk)
- S + O2 → SO2
- What term is given to the temperature of 960C shown above?(*1mark)
- transitional temperature
- Identify the allotropes:
-
- Name substance: X and V( 2 mks)
- X; oxygen
- V;vanadium (V)oxide
- What is the role of the following substances ?
- Solid V(1 mk)
- act as a catalyst
- Fused calcium chloride(1mk)
- prevent entry of moisture into the flask
- Salt in the Ice + salt mixture(1mk)
- salt act as an impurity to lower melting point of ice
- Solid V(1 mk)
- Explain why the fume chamber is used?(1mk)
- to absorb excess poisonous sulphur (V) oxide
- Write an equation for the reaction that took place in the combustion tube.(1mk)
- SO2+O2 → SO3
- Name substance: X and V( 2 mks)
- Starting with zinc metal, describe how a solid sample of zinc hydroxide can prepared.(3 marks)
- To nitric acid add excess zinc metal, filter to remove excess zinc. Add sodium hydroxide to zinc nitrate solution.
- A precipitation reaction will occur where zinc hydroxide will be precipitated out. Filter to obtain zinc hydroxide as the residue and dry it between filter paper.
- The substances and apparatus below were used to test the presence of nitrate in substance D.
- Identify substance D (1 mark)
- Concentrated sulphuric acid
- What are the components of the brown ring.(1 mark)
- Iron (II) sulphate-nitrogen (II) oxide complex
- Identify substance D (1 mark)
- Nitrogen does not support combustion yet burning magnesium introduced into a gas jar of nitrogen continues to burn, forming a white solid. Explain.(1 mark)
- Burning magnesium produce a lot of heat capable of breaking triple bond holding nitrogen atoms together
- Write an equation for the reaction forming the white solid.(1 mark)
- Mg + N2 → Mg3N2
- State two uses of nitrogen.(1 mark)
- Haber process
- Manufacture of nitrogenous fertiliser
- Write an equation for the reaction forming the white solid.(1 mark)
- Study the flow chart below and answer the questions that follow.
Identify- Solution K.(1 mark)
- Nitric acid
- Solid L(1 mark)
- PbO
- gas M ( 1 mk)
- Oxygen gas
- Solution K.(1 mark)
- Study the scheme below and answer the questions that follow.
Explain the observations made in- Step 1 (1 mark)
- Low viscosity and flows easily
- Step 2 (1 mark)
- Liquid sulphur becomes very viscous
- Step 3 (1 mark)
- Turns black and flows easily
- Step 1 (1 mark)
- Study the diagram below and use it to answer the questions that follow.
- Identify electrodes.(2 marks)
- A; anode
- B; cathode
- Name the product formed at the anode.(1 mark)
- Bromine gas
- Identify electrodes.(2 marks)
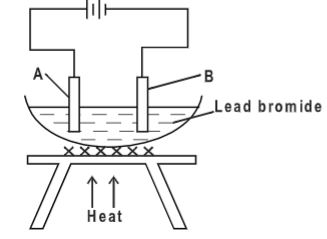
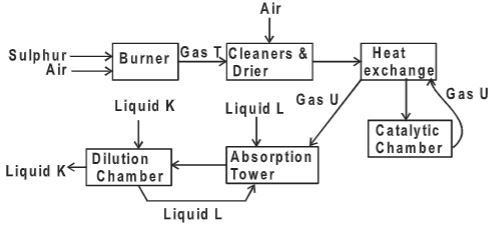
- Th ane flow diagram below is a summary of the industrial manufacture of sulphuric (VI) acid.
- Write an equation for the reaction in the burner.(1 mark)
- S + O2 → SO2
- Why is it important to pass gas T and air through cleaners?(1 mark)
- To remove dust which might reduce efficiency of catalyst
- Identify
- Gas U (½ mark)
- SO3
- Liquid K(½ mark)
- water
- Liquid L(½ mark)
- Sulphuric acid
- Gas U (½ mark)
- Write equation for the reaction taking place in the catalytic chamber (1 mark)
- SO2 + O2 → SO3
- Name the most suitable catalyst that can be used in the catalytic chamber. (1 mark)
- Vanadium(V)oxide
- Give the name of the product formed in the absorption tower.(1 mark)
- oleum
- Write equation for the reaction taking place in the dilution chamber. (1 mark)
- H2S2O7 +H2O → 2H2SO4
- Name the main pollutant in this process and state how it is taken care of.(1½ marks)
- SO2, is passed through a chimney lined with calcium hydroxide
- Give one use of sulphuric (VI) acid.(1 mark)
- manufacture of fertiser, manufacture of plastics, manufacture of detergents
- Write an equation for the reaction in the burner.(1 mark)
Join our whatsapp group for latest updates
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Download Chemistry Questions and Answers - Form 3 Mid Term 2 2022.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students