SECTION A: BIOLOGY (34 marks)
Answer ALL the questions in this section in the spaces provided
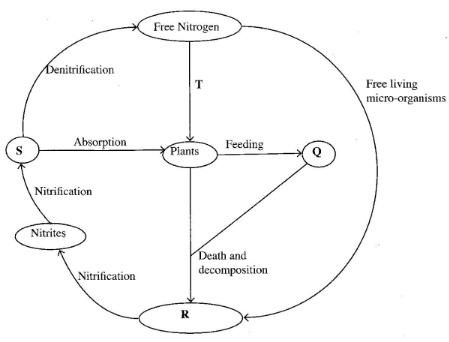
- The diagram below represents the nitrogen cycle:
- Name the components labelled Q, R and S (3 marks)
- Name the process labelled T. (1 mark)
- Give one example of organisms that cause decomposition. (1 mark)
-
- State one function of each of the following structures in the human reproductive system:
- ovary: (1 mark)
- epididymis. (1 mark)
- What is gestation period? (1 mark)
- State one function of each of the following structures in the human reproductive system:
-
- State the meaning of the following terms:
- growth; (1 mark)
- development. (1 mark)
- What is the importance of dormancy in seeds? (3 marks)
- State the meaning of the following terms:
- State two differences between continuous and discontinuous variations (2 marks)
-
-
- What is natural selection? (1 mark)
- Give one example of natural selection. (1 mark)
- State one adaptation of Ascaris lumbricoides that enables them survive the digestive enzymes of their host. (1 mark)
-
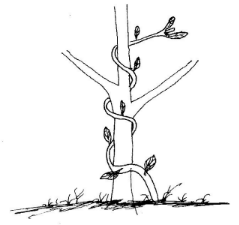
- The diagram below illustrates a certain tropic response.
- Name the tropic response illustrated in the diagram. (1 mark)
- Give two survival values of the tropic response shown above to the plant. (2 marks)
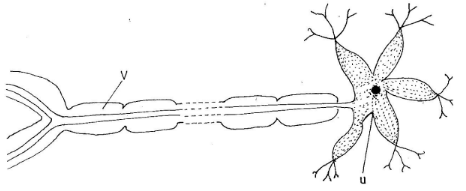
- The diagram below represents a neurone.
-
- Name the part labelled V. (1 mark)
- State one adaptation of the part labelled U to its function. (1 mark)
- Name the part of the ear that is responsible for balancing. (1 mark)
-
-
- Name two types of movable joints in human beings. (2 marks)
- State one function of the parenchyma tissue in young plants. (1 mark)
-
- What does the term implantation mean in human reproduction? (1 mark)
- State two ways of reducing the spread of herpes simplex. (2 marks)
- Why is the sex of a child determined by the father and not the mother? (4 mark)
SECTION B: CHEMISTRY (33 marks)
Answer ALL the questions in this section in the spaces provided.
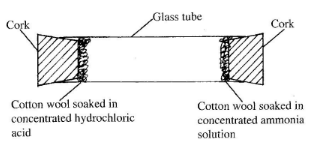
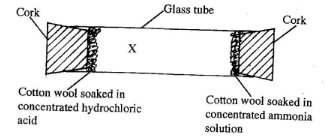
- The set-up shown below was used to investigate the rate of diffusion of ammonia and hydrogen chloride gases. Study it and answer the questions that follow.
- State the observation made in the glass tube. (1 mark)
-
- On the diagram, indicate with a cross (X) the likely position where the above observation is made. (1 mark)
- Explain your answer in b(i) above. (1 mark)
- Calculate the mass that is contained in 0.1 moles of calcium carbonate. (Ca = 40.0: C = 12.0; 0 - 16.0).(2 marks)
- In trying to investigate some properties of chlorine gas, a student introduced wet blue litmus paper into a gas jar containing the gas.
- State the observations made in the gas jar. (1 mark)
- Explain the observations in (a) above. (1 mark)
-
-
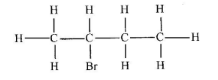
- Name the compound whose structure is given below: (1 mark)
- Draw the structure of pent-2-ene. (1 mark)
- Name the compound whose structure is given below: (1 mark)
- Describe a chemical test that can be used to distinguish between butane and but-l-ene. (2 marks)
-
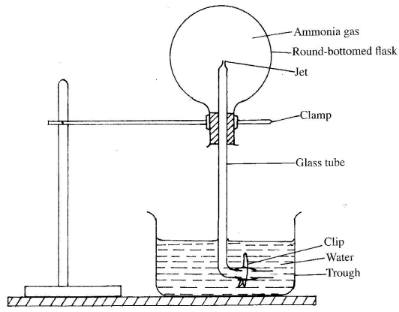
- The set-up below was used to investigate some properties of ammonia gas. Study it and answer the questions that follow.
- The clip was slightly opened to allow a drop of water to move up to the top of the glass tube. After a few minutes, the clip was opened to allow more water to pass.
- State the observation made in the round-bottomed flask. (½ mark)
- Explain the answer in ai) above. (1½ marks)
- Name two chemicals that can be used to prepare ammonia gas in a school laboratory, (1 mark)
- Give two commercial uses of ammonia. (1 mark)
- The clip was slightly opened to allow a drop of water to move up to the top of the glass tube. After a few minutes, the clip was opened to allow more water to pass.
- Sulphur (IV) oxide and oxygen react as shown in the equation below.
2SO2(g) + O2(g)2SO3(g) ; ΔH = -ve
- What is meant by ΔH = -ve? (1 mark)
- Explain the effect of increasing pressure on the position of equilibrium of the above reaction.
(2 marks) - Give one use of sulphur (VI) oxide. (1 mark)
- Charcoal and kerosene are some of the fuels commonly used in Kenyan homes.
- What is meant by the term fuel? (1 mark)
- Write a chemical equation for the complete combustion of charcoal. (1 mark
- Give two advantages of using kerosene as a fuel over charcoal. (2 marks)
- Name two sources of energy in Kenya that are environmentally friendly. (1 mark)
- 142g of sodium sulphate were dissolved in 200 cm3 of distilled water. More water was added to make up to 500 cm3 of solution.
- Calculate the molarity of the solution formed (Na = 23.0; S = 32.0;0 - 16.0). (2 marks)
- What volume of the solution is required to make a litre of solution of 0.5M. (2 marks)
-
- The raw materials used in the extraction of iron are iron ore, calcium carbonate, coke and air. (1)
- Write an equation for a reduction process in the blast furnace if the ore used was iron (III) oxide. (1 mark)
- What is the purpose of the calcium carbonate? (1 mark)
- Explain how the silica impurities are removed from the blast furnace. (2 marks)
- Give one alloy that contains iron. (1 mark)
- The raw materials used in the extraction of iron are iron ore, calcium carbonate, coke and air. (1)
SECTION C: PHYSICS (33 marks)
Answer ALL the questions in this section in the spaces provided
- An object of height 24 cm is placed in front of a concave mirror. The magnification of the image is 0.5. Determine the height of the image. (3 marks)
- It is observed that when a glass rod is brought near a positively charged sphere, repulsion occurs. State a reason for the repulsion. (1 mark)
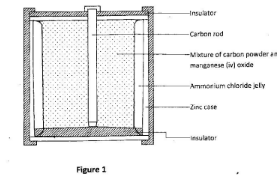
- Figure 1 represents a dry lechianché cell.
State the use of:- carbon powder; (1 mark)
- mangenese (IV) oxide (1 mark)
- In a laboratory there are two soft iron bars and two bar magnets. In the space provided draw a diagram to show how the two bar magnets and the soft iron bars can be arranged so that the strength of the magnet is maintained for a long time. (1 mark)
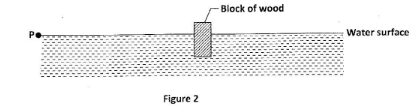
- Figure 2 shows a block of wood floating in water.
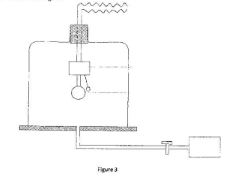
A wave is generated at point P. After some time the block of wood is seen to move up and down. State, with a reason the type of wave formed in the water. (2 marks) - Figure 3 represents a set up that is used to study a property of sound. The pump in the set up is used to remove air from the jar.
As the air was being removed from the jar the loudness of the sound of the bell decreased until the sound could no longer be heard. Explain why the sound could no longer be heard although the bell continued working. (2 marks) -
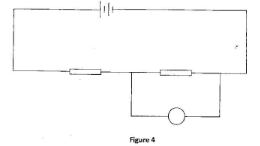
- Figure 4 shows a battery whose potential difference is 3 V connected in series with resistors R1 and R2 A voltmeter V is connected across R2
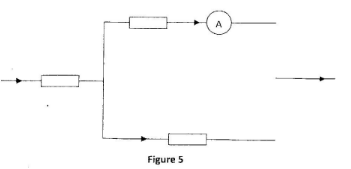
The potential difference across R is 2 V. Determine the reading of the voltmeter. V. (1 mark) - Figure 5 shows part of a circuit containing three resistors R3, R4 and R5 and an ammeter. A current of 0.4 A is flowing through R2 and a current of 0.1 A is flowing through R4
Determine the reading on the ammeter. (1 mark)
- Figure 4 shows a battery whose potential difference is 3 V connected in series with resistors R1 and R2 A voltmeter V is connected across R2
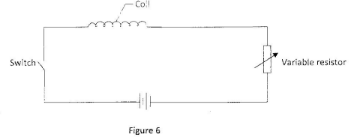
- Figure 6 shows a circuit in which a coil of wire is connected in series with a variable resistor, a battery and a switch.
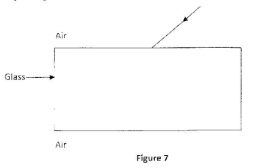
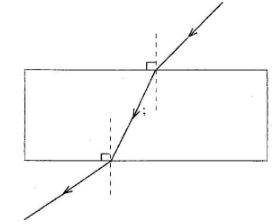
The coil gets heated when the switch is put on. The resistance in the circuit is then reduced using the variable resistor. State, with a reason the effect on the heat produced in the coil (2 marks) - Figure 7 shows a ray of light in air incident to a rectangular glass block.
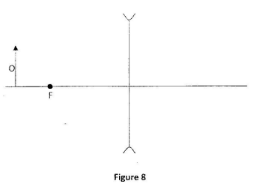
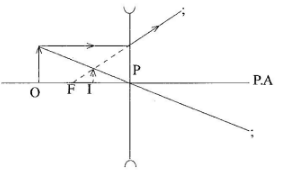
Complete the diagram to show the path of the ray as it passes through the glass into the air (2 marks) - Figure 8 shows an object in front of a diverging lens. The principal focus of the lens is marked F.
On the figure, draw a ray diagram to locate the image. (3 marks) - Figure 9 shows a graph of amplitude against time for a wave.
Using the figure, determine the period of the wave. (1 mark) -
- Explain why the voltage of the mains electricity from a generating station is stepped up before the electricity is transmitted over long distances. (2 marks)
- State why a fuse should be connected to the live wire in a domestic wiring circuit (1 mark)
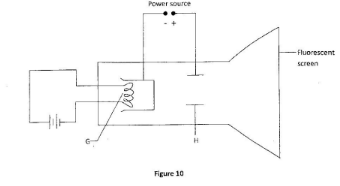
- Figure 10 shows a simplified cathode ray tube.
- Name the part labelled H. (1 mark)
- State the purpose of the part labelled G. (1 mark)
- State how the cathode rays affect the screen. (1 mark)
-
- An X-ray tube produces X-rays of low penetrating power. State the adjustment that should be made to the circuit connected to the tube so that the X-rays produced are of a higher penetrating power.
(1 mark) - State a reason why X-rays are not deflected when they pass through an electric field. (1 mark)
- An X-ray tube produces X-rays of low penetrating power. State the adjustment that should be made to the circuit connected to the tube so that the X-rays produced are of a higher penetrating power.
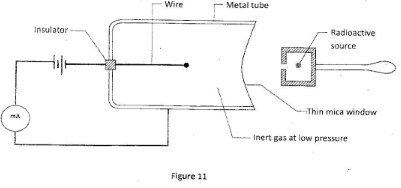
- Figure 11 shows an instrument which is used to detect radioactive emissions. The metal tube contains an inert gas at low pressure.
When the radioactive source is brought close to the mica window the milliammeter is observed to deflect repeatedly. Explain this observation. (2 marks) - Explain how an n-type semiconductor can be produced from silicon (2 marks)

MARKING SCHEME
SECTION A: BIOLOGY
-
- Q - Animals
R - Ammonia/NH4;
S - Nitrates: (3 marks) - Nitrogen fixation; (1 mark)
- Fungi/saprohytic organisms; Bacteria;
(any one correct) (1 mark)
- Q - Animals
-
-
- Produce ova; produce hormones;
(any one correct) (1 mark) - Temporary storage of sperms; place where sperms develop motility:
(any one correct) (1 mark)
- Produce ova; produce hormones;
- The time between fertilization and birth. (1 mark)
-
-
-
- Growth is quantitative increase in size which is permanent;
- Development is qualitative changes involving differentiation; to form tissues. (1 mark)
-
- To survive adverse conditions:
- To allow dispersal;
- To allow embryo to mature; (3 marks)
-
-
- Continuous variation has intermediates for a particular characteristic while discontinuous variation has no intermediates: (1 marks)
- Continuous variation is influenced by both genes and environment while discontinuous variation is influenced by genes only; (1 mark)
-
-
- Organisms with favourable variations survive and reproduce while those with unfavourable variations reduce in numbers/become extinct (1 mark)
-
- Industrial melanism/peppered moth;
- Resistance to drugs/pesticides/antibiotics:
- (any one correct) (1 mark)
- Thick cuticle: secretion of antienzymes/mucus;
(any one correct) (1 mark)
-
-
- Thigmotropism/Haptotropism; (1 mark)
- Support:exposure to light; (2 marks)
-
-
- Myelin sheath: (1 mark)
- U-has dendrites which receive impulses from other neurones: (1 mark)
- Semi-circular canals: (1 mark)
-
-
-
- hinge joints;
- ball and socket joints:
- gliding joints;
- pivot joint:
(first two correct) (2 marks)
-
- Packing:
- mechanical support;
(first one correct) (1 mark)
-
-
- Attachment of zygote to the wall of the uterus; (1 mark)
-
- Avoid indiscriminate sex/kissing:
- Avoid sharing of needles and syringes: (2 marks)
-
- Father produces two types of gametes/sperms X and Y:
- Mother produces only one type of gamete/ova X;
- When an ovum is fertilized by the Y sperm, a boy results:
- An ovum fertilized by the X sperm forms a girl;
(4 marks)
SECTION B: CHEMISTRY (33 Marks)
-
- A white ring is formed in the glass tube. (1 mark)
-
- The cross (X) should be nearer to the source HCI (g). (1 mark)
- Since ammonia (RMM =17) is less dense than HCl gas (RMM = 36.5), it will diffuse faster than HCI, (1 mark)
- The cross (X) should be nearer to the source HCI (g). (1 mark)
- CaCO3✓= 40 + 12 + 48 = 100✓
0.1X100 = 10g (2 marks)
1 -
- Blue litmus paper will turn to red and then bleached/turns white. (1 marks)
- Litmus paper turned to red because chlorine is acidic and then decolourised/turned white because the gas is a bleaching agent. (1 mark)
-
-
- 2 - bromobutane (1 mark)
-
(1 mark)
- Place acidified potassium manganate (VITY/bromine water in separate test tubes. Bubble the gases separately through the solutions. With but-1-ene, the two solutions will be decolourised while butane will not decolourise both solutions. (2 marks)
-
-
-
- The water comes out inform of a fountain". (½ mark)
- This is due to the partial vacuum ✓½ that is created in the flask as a lot of the ammonia gas dissolves ✓½ in the first drop of water and the water is forced rapidly up the tube and enters the flask as foutain. ✓½ (1½ marks)
- Ammonium chloride salt (NH4Cl)
Calcium hydroxide (Ca(OH)2) (1 mark)- Bubble but-1-ene and butane through separate test tubes containing acidified potassium manganate (vii). Acidified KMnO4 will turn from purple to colourless with butane.
- Bubble but-1-ene and Butane through separate test tubes containing bromine water. Bromine water is decolourised by but-1-ene but it remains brown with butane.
But-l-ene burns with sooty luminous flame but butane bums with blue non-luminous flame. Bubble but-1-ene and butane through separate test tubes containing acidified potassium dichromate (VI).
But-l-ene turns acidified potassium dichromate (VI) from orange to green but remains orange with butane.
-
- Large quantities of ammonia gas used to make fertilizers
- Liquid ammonia used as a refrigerant
- Ammonia solution is used as a solvent in laundry
- Manufacture of ammonia salts.
- Ammonia gas used in manufacture of nitric (V) acid.
- Manufacture of dyes and fibres.
- Manufacture of fibres.
- Used to soften hard water.
(Any two correct) (1 marks)
-
-
- the reaction is exothermic. (1 mark)
- The equilibrium will shift to the right since the volume of product is less than that of reactants.
(2 marks) -
- Purifying petroleum products
- Manufacture of sulphuric (VI) acid
- Bleaching fumigant and as food preservative.\
(Any one correct)(1 mark)
-
- A fuel is a material that releases heat energy when burned. (1 mark)
- C(s) + O2(g) → CO2(g) (1 mark)
-
- High heat content
- Does not lead to deforestation
- Easy to transport
- Cleaner fuel than charcoal.
- Easier to ignite
- Solar, Geothermal, wind, hydroelectricity & tidal waves.
(Any two correct marks) (2 marks)
-
- Na2SO4 RFM = (23 x 2) + 32 +(16 x 4)
= 46 + 32 +64 = 142 ✓½
= 142 = 1 mole ✓½
142
500cm3 contains 1 mole
1000cm3 would contain ?
1000 x 1 ✓½
500
= 2 M ✓½ (2 marks) - M1V1 = M2V2
2 x V1 =0.5 X 1000 ✓½
V1 = 0.5 x 1000 ✓½ = 250 cm ✓1 (2 marks)
2
- Na2SO4 RFM = (23 x 2) + 32 +(16 x 4)
-
-
- Fe2O3(s) + 3CO(g) → 2Fe(l) +3CO2(g) (1 mark)
- Decomposes to give carbon (IV) oxide and calcium oxide which are both used in the process.(1 mark)
- Calcium oxide react with silica to give calcium silicate (slug) which form a liquid layer on top of liquid iron as it flows away. (2 marks)
- Steel (1 mark)
-
SECTION C: PHYSICS (33 Marks)
- Magnification = Image height =0.5
object height
Image height = 0.5 x object height
= 0.5 x 24 cm;
= 12 cm; (3 marks) - The glass rod is positively charged; (1 mark)
- carbon powder - to increase conductivity between the carbon rod and the zinc case: (1 mark)
- manganese IV oxide-a depolarizer, (1 mark)
-
(1 mark)
-
- Transverse wave;
- Movement of the block is perpendicular to the direction of the wave motion: (2 marks)
- A vacuum was created by pumping the air out of the jar, Sound requires a material medium for propagation; (2 marks)
-
- IV:
- 0.3 A; (2 marks)
-
- Heat will increase;
- Reducing resistance increases the current;
(2 marks)
-
- Refracted Ray Bending Towards Normal;
- Emerging ray bending away from normal; (2 marks)
-
- Ray from O parallel to PA then from lens;
- Ray from through pole P;
- Image erect virtual at intersection of they rays
(3 marks)
- Periodic time=0.4 seconds; (1 mark)
-
- Stepping up reduces current of transmission: hence reducing heat loss; (2 marks)
- To isolate all parts which are connected to the live wire: When there is excess current. (1 mark)
-
- Anode;
- To head the cathode:
- The screen glows: (3 marks)
-
- Increase the anode voltage:
- X-rays have no charge, (2 marks)
- Radioactive emission enters the tube and causes ionization of the gas inside the tube. Opposite charges are attracted to opposite electrodes creating a current: (2 marks)
- By doping: with Group 5 element; (2 marks)
Download KCSE 2012 General Science Paper 2 with Marking Scheme.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students