SECTION A: BIOLOGY (34 marks)
Answer ALL the questions in this section in the spaces provided.
-
- Name one example of an organism in the kingdom monera.
- State one function for each of the following parts of a light microscope:
- coarse adjustment knob;
- diaphragm.
-
- Figure 1 represents a mammalian heart.
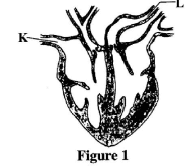
On the figure, draw arrows to show the direction of flow of blood in vessels labelled K and L. (2 marks) - Students carried out an experiment in which two strips of cobalt chloride paper were clipped one on the upper surface and the other on the lower surface of a leaf of a terrestrial plant. The experimental set-up was left for sometime.
- On which side of the leaf did the cobalt chloride paper change colour faster? (1 mark)
- Give a reason for your answer in (b) (i) above. (1 mark)
- Figure 1 represents a mammalian heart.
- Figure 2 illustrates a set-up that was used to demonstrate a process that takes place when animal cells are placed in a hypertonic solution.
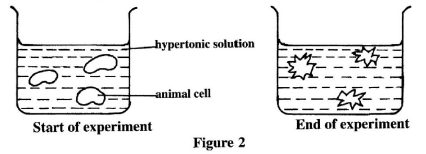
- Describe the shape of the cells at the end of the experiment. (1 mark)
- Explain the observation made at the end of the experiment.(2 marks)
-
- State two factors that determine energy requirements in human beings.(2 marks)
- Distinguish between autotrophic and heterotrophic nutrition.(2 marks)
-
- State two differences between aerobic and anaerobic respiration. (2 marks)
- Name the branch of biology that deals with the study of relationships between organisms and their environment. (1 mark)
-
- State two features in lungs that make them suitable for gaseous exchange. (2 marks)
- State one symptom of asthma. (1 mark)
-
- Figure 3 represents a transverse section of a mammalian skin.
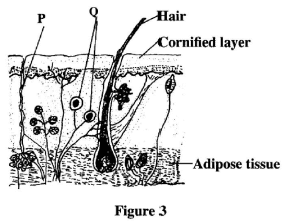
Name the structures labelled P and Q.- P ............(1 mark)
- Q ........... (1 mark)
- Explain how the kidney regulates the amount of water in the body. (2 marks)
- Figure 3 represents a transverse section of a mammalian skin.
-
- State two ways of controlling liver cirrhosis. (2 marks)
- What is excretion? (1 mark)
-
- State the meaning of the following terms:
- tissue;(1 mark)
- cell. (1 mark)
- State one importance of scientific naming of organisms. (1 mark)
- State the meaning of the following terms:
-
- State ways by which mineral salts and water are absorbed into the root hairs of plants.
- Mineral salts;(1 mark)
- Water. (1 mark)
- Explain how one can determine if a food substance contains proteins. (2 marks)
- State ways by which mineral salts and water are absorbed into the root hairs of plants.
SECTION B: CHEMISTRY (33 marks)
Answer ALL the questions in this section in the spaces provided.
- Table 1 shows the pH values of solutions P, Q, R and T.
Table 1
Solution P Q R T pH 9 6 11 2 - State the observations made when both blue and red litmus papers are dipped into solution P. (1 mark)
- Name the type of reaction that occurs when solution R reacts with solution T. (1 mark)
-
- Given the following reagents; lead (II) oxide, dilute nitric (V) acid and sodium sulphate solution, describe how a student can prepare a sample of lead (II) sulphate. (3 marks)
- State one use of calcium sulphate salt. (1 mark)
- Figure 4 shows a set-up that was used to investigate the properties of some of the components of air.
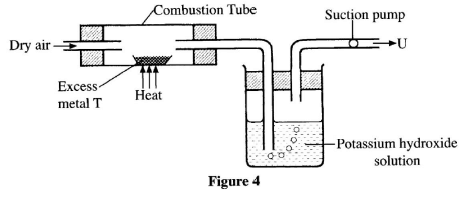
- Metal T is in group two of the periodic table. Write the formula of the product formed in the combustion tube. (1 mark)
- State the role of potassium hydroxide solution. (1 mark)
- Identify the main gas in U. (1 mark)
-
- An atom of element D has a mass number of 19 and 10 neutrons.
- Write the electron arrangement of the atom. (1 mark)
- In which period does element D belong? (1 mark)
- A compound has a formula X, Y,. In which group of the periodic table does X belong? (1 mark)
- An atom of element D has a mass number of 19 and 10 neutrons.
- The elements L, M and N are in the same group. Study Table 2 and answer the questions that follow. The letters are not the actual symbols of the elements.
Table 2
Element Atomic number Atomic radius (nm) First ionization energy in kJmol-1 L 4 0.089 900 M 12 0.136 736 N 20 0.174 590 -
- Explain why the atomic radius of N is larger than that of L. (1 mark)
- Arrange the elements in order of reactivity starting with the least reactive. (1 mark)
- Why does the 1st ionisation energy of the elements decrease down the group? (2 marks)
-
-
- A student used the apparatus in Figure 5 to separate liquids L, and L.
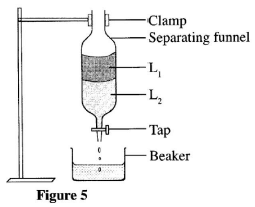
State two physical properties that enabled liquids L, and L, to be separated. (2 marks) - What precaution should be taken to ensure complete separation of the two liquids? (1 mark)
- A student used the apparatus in Figure 5 to separate liquids L, and L.
-
- Explain why diamond is the hardest substance. (2 marks)
- State one use of diamond. (1 mark)
- The set-up shown in Figure 6 was used by a student to investigate the effect of electric current on zinc nitrate crystals.
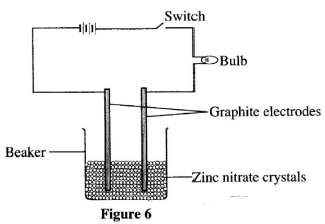
- When the switch was put on, the bulb did not light. When water was added into the beaker, the bulb lit. Explain. (2 marks)
- Identify the ions that were attracted to the cathode.(1 mark)
-
- Describe how temporary hard water is formed. (2 marks)
- State one advantage of drinking hard water. (1 mark)
- Study the set-up shown in Figure 7 and answer the questions that follow.
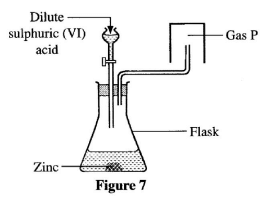
- Write an equation for the reaction that occurs in the flask. (1 mark)
- Explain why the gas is collected as shown in the set-up. (1 mark)
- State one commercial use of gas P. (1 mark)
- State two physical properties that are used to determine purity of a substance. (2 marks)
SECTION C: PHYSICS (33 marks)
Answer ALL the questions in this section in the spaces provided.
- A stopwatch used to time a falling object started 0.20 s after the start button was pressed. The time recorded was 3.22 s, determine the time of the fall. (2 marks)
- Describe adhesive forces. (2 marks)
- It was observed that a partially inflated balloon becomes fully inflated as it rises. Explain this observation. (2 marks)
- Fine chalk dust particles are suspended in water then observed through a microscope. The particles are observed to move in a random manner. Explain this observation. (2 marks)
- Name two types of thermometers. (2 marks)
- Explain why a metallic seat inside a room feels cooler than a wooden seat in the same room. (2 marks)
- Ventilation holes in a room are placed at a higher level than the doors and windows. Explain how they work to keep the room ventilated. (2 marks)
-
- Define the term displacement as used in linear motion. (1 mark)
- Figure 8 represents a displacement-time graph of a body in motion.
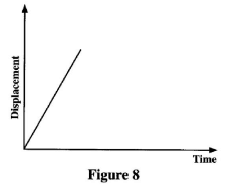
Describe the motion of the body. (2 marks)
- Figure 9 shows a set-up that was used to determine the weight (w) of a wooden plank.
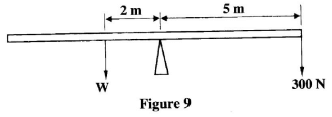
Calculate the value of w. (3 marks) - A cork with a narrow tube through it is used to seal a conical flask full of water at 0°C. The water level in the tube is above the cork. The flask is held vertically and lowered into hot water. Explain what is observed. (2 marks)
- State the energy changes that take place when a stone is thrown vertically upwards, reaches the highest point then starts falling. (2 marks)
- A student observed that a string extended by 3 cm when supporting a load of 4.5 N. Determine its spring constant. (3 marks)
- Define the term centre of gravity. (1 mark)
- A bag of maize at the back of an open pick-up slides backward when the pick-up suddenly starts moving forward. Explain the reason why this happens. (2 marks)
- A piece of steel sinks in water. Explain how it can be made to float. (3 marks)

MARKING SCHEME
SECTION A: BIOLOGY
-
- Bacteria; (1 mark)
-
- Coarse adjustment knob - raising and or lowering the body tube to bring image into sharp focus; (1 mark)
- Diaphragm - regulate the amount of light entering the condenser; (1 mark)
-
- K - brings blood into the heart;(1 mark)
L - takes blood away from the heart;(1 mark) -
- On the lower surface; (1 mark)
- The lower surface has more stoma than the upper surface; (1 mark)
- K - brings blood into the heart;(1 mark)
-
- Crenated/shrinked/shrunken; (1 mark)
- Water moves from cell to (the hypertonic) solution by osmosis; cell membrane shrinks losing its original shape (becomes crenated); (2 marks)
-
- Age;
Basal metabolic rate;
Occupation/ everyday activity;
Sex, Body size;
State of health; (2 marks) - Autotrophism - simple materials are built up/synthesised to form complex food substances;
Heterotrophism - complex food substances are broken down to simple food substances
(2 marks) Mark as a whole
- Age;
-
(2 marks)Aerobic respiration Anaerobic respiration Oxygen is used;
Water and energy are produced;
More energy is released;
Takes place in cytoplasm and mitochodrian;Oxygen is not used;
Alcohol, lactic acid and energy are produced;
Less energy is produced;
Takes place in cytoplasm;- Ecology; (1 mark)
-
-
- Lined with a thin membrane/ one-cell thick layer;
- Moist;
- Highly vascularised;
- Numerous alveoli; (2 marks)
- Overproduction of mucus;
Production of hissing sound - while breathing;
Low blood pressure; (1 mark)
-
-
- P-sweat duct;
Q- temperature/ pressure receptor; (2 marks) - High osmotic pressure of blood leads to production of more anti-diuretic hormone (ADH); making more water to be reasorbed from the kidney tubules into the blood-stream; low osmotic pressure of blood leads to production of less ADH; making less water to be reabsorbed from the kidney tubules into the blood stream. (2 marks)
- P-sweat duct;
-
- Avoid excessive intake of alcohol; Timely treatment of liver infections; (2 marks)
- Removal of metabolic waste from the body; (1 mark)
-
-
- Tissue - group of cells specialised to perform a given function;
- Cell - structural and functional unit of living organisms; Basic functional (2 marks)
- Universal identity to avoid confusion; They rarely change; (1 mark)
-
-
-
- active transport;
- osmosis; (2 marks)
- To the solution of the food substance add an equal amount of sodium hydroxide solution and a drop of Copper (II) Sulphate solution/Biuret reagent; a purple colour indicates presence of protein while a blue colour shows absence;
(Correct procedure and reagents) (2 marks)
-
SECTION B: CHEMISTRY
-
-
- Blue litmus paper remains blue.
- Red litmus paper turns to blue. (1 mark)
- Neutralisation (1 mark)
-
-
-
- Add dilute nitric (V) acid to excess lead (II) oxide, to get a solution of lead (II) nitrate
- filter to collect filtrate of lead (II) nitrate
- Add sodium sulphate solution to the filtrate;
- Filter to obtain the residue;
- Rinse and dry residue between litmus papers (3 marks)
-
- manufacture of cement
- plastering walls
- manufacture of plaster of paris
(Any one correct)
(1 mark)
-
-
- TO(1 mark)
- It absorbs/reacts with carbon (IV) oxide gas(1 mark)
- Nitrogen gas (1 mark)
-
-
- mass number - neutrons = (19 - 10) = 9 electrons
Electron arrangement - 2.7 (1 mark) - period 2 (1 mark)
- mass number - neutrons = (19 - 10) = 9 electrons
- Group 3 (1 mark)
-
-
-
- Its larger because more energy levels are added down the group hence increasing the atomic radius of element N away from the nucleus. (1 mark)
- L, M, N. (1 mark)
- As more energy levels are increased down the group, the electrons on the outer most energy level become less attracted to the positive nucleus, hence less energy is required to remove the first electron. (2 marks)
-
-
- Density of the liquids
The molecules of the two liquids don't mix to form homogeneous solution, liquids are immiscible. (2 marks) - Discard the liquid where solution L1 and L2 meet.
OR
Run L2 into a beaker and discard a small amount at interface. (1 mark)
- Density of the liquids
-
-
- All the four electrons in diamond are used in forming covalent bonds vi
- The structure of diamond is tetrahedral and cross linked (2 marks)
-
- Drilling rocks
- Jewellery (Any one correct) (1 mark)
-
-
-
- The bulb did not light initially because the ions were immobile;
- When water is added, the crystals dissociate to form ions which are free to move and conducts the electricity.(2 marks)
- H+ , Zn2+ (1 mark)
-
-
-
- Carbon (IV) oxide in the air dissolves in rain water from the atmosphere to form carbonic acid (H,CO2);
- The weak carbonic acid reacts with rocks containing calcium carbonate and magnesium carbonate to form calcium hydrogen carbonate and magnesium hydrogen carbonate which causes temporary hardness of water. (2 marks)
- Calcium salts in hard water are used in the formation of bones and teeth. (1 mark)
-
-
- Zn(s) + H2SO4(aq) -> ZnSO4(aq) + H2(g) (1 mark)
- The gas is lighter/less dense than air (1 mark)
-
- filling balloons for weather studies
- manufacture of ammonia
- manufacture of margarine
- rocket fuel
- Manufacture of hydrochloric acid
(Any one correct) (1 mark)
-
- boiling point
- melting point
- density
- refractive index
- viscosity
(Any two correct) (2 marks)
SECTION C: PHYSICS
- 3.22 +0.20 = 3.245
- Adhesive forces are forces of attraction between molecules of different types substances/ kind. Cohesive forces are forces of attraction between molecules of similar type
- As the balloon rises the atmospheric pressure decreases and thus the air/gas in the balloon expands/pressure in.
- The movement is caused by collision of the dust particles by the water particles; which are in continuous/constant random motion.
- clinical thermometer
ordinary thermometer
maximum - minimum thermometer/six's thermometer
liquid in glass thermometers
bimetallic thermometer
gas thermometer
eletronic thermometer - Metal are better conductors of heat than wood. Thus when touched metals conduct heat away (from body) faster than wood which is a poor conductor/ metals are good conductors while wood is a poor conductor/insulator; metals conduct heat away faster.
- Warm air rises and escapes through the ventilations while cool air comes in through the doors and windows or warm air is less dense than cold air and rise cold air take its' place.
-
- This is the distance between two points in a specified direction. A vector quantity has both magnitude and direction /distance in a specified direction.
- The body is moving with uniform velocity in a positive direction.
- Clockwise moments = Anti clockwise moments
300 x 5 = 2 x W
W = 750 N - The level of the water in the tube first drops then rises up. as the flask first expands then the water starts to expand. Water expands more than the flask.
- Kinetic → Potential → Kinetic
- F = ke
4.5 = 3K
K=1.5 N/cm - The point through which the resultant of the weights of all the particles of a body acts or appears to act. Point of application of the resultantforce due to the earth's pull on a body.
- This is because of inertia. The bag tends to remain stationary as the pick-up moves forward.
- Change the shape of steel to increase the upthrust (volume displaced). This lowers the average density of the steel. Weight of steel equals upthrust.
Download KCSE 2015 General Science Paper 1 with Marking Scheme.
Tap Here to Download for 50/-
Get on WhatsApp for 50/-
Why download?
- ✔ To read offline at any time.
- ✔ To Print at your convenience
- ✔ Share Easily with Friends / Students

